Karin Stiasny, Simon Raffl, Stephan W. Aberle, Judith H. Aberle
ECDC risk status: endemic
(last edited in May 2025, update for 2024: 158 reported cases)
History and current situation
Since 1972, the documentation of human cases of tick-borne encephalitis (TBE) in Austria has been performed by the Center for Virology, Medical University of Vienna, which acts as the National Reference Laboratory for TBE and other flavivirus infections. Only hospitalized patients with a recent tick-borne encephalitis virus (TBEV) infection confirmed by laboratory diagnosis are counted as cases. Confirmation is usually based on immunoglobulin (Ig) serology (namely enzyme-linked immunosorbent assay [ELISA] for IgM and IgG). However, this confirmation may be supplemented by virus neutralization and polymerase chain reaction (PCR) analyses if needed.
In 2012, TBE became a notifiable disease in Austria as in other countries of the European Union.1 The annual incidence rates of TBE in Austria have declined substantially since the 1980s.2 This decline was associated with an increasing rate of vaccination and was not observed in some neighboring countries, for example, Czech Republic and Slovenia, where vaccination coverage is much lower than in Austria.2
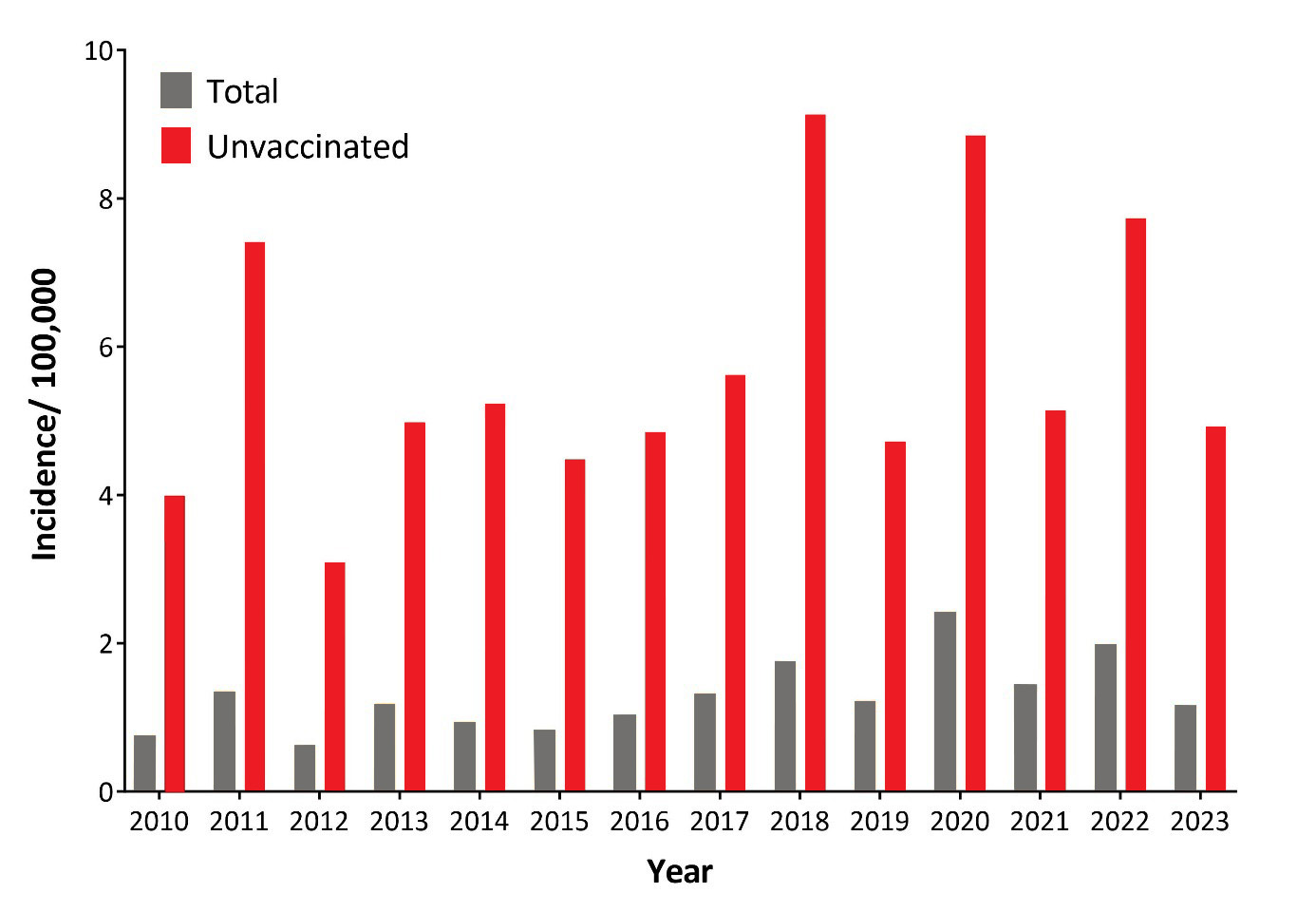
Incidences of TBE in the total and unvaccinated population in Austria from 2010 to 2023 are shown in Figure 1. Strong annual fluctuations are a characteristic feature of the epidemiology of TBE in Austria, indicating a complex interplay of factors that control viral transmission dynamics in natural hosts and human risk exposure. The age distribution of TBE incidences in Austria is strongly shifted towards older people2 and reveals a peak in the population 41 to 80 years of age (Figure 2). In addition to virus transmission by tick bites, alimentary infections through the consumption of infected goat cheese have been documented.3,4 TBE viruses isolated in Austria from ticks and humans were shown through molecular analyses to be members of the European subtype of TBEV (TBEV-Eu)5 (and Gerhard Dobler, personal communication; Stephan W. Aberle and Jeremy V. Camp, unpublished results).
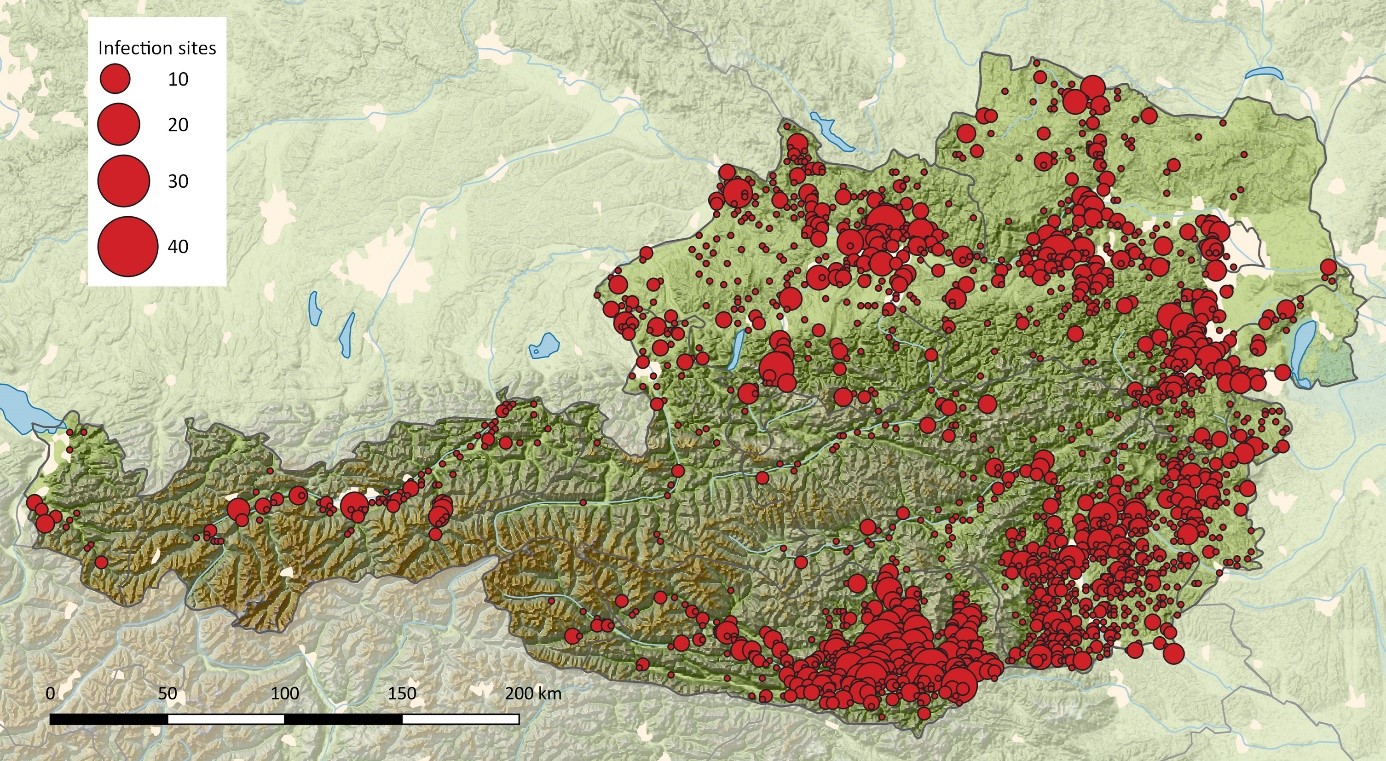
Mapping of the most likely sites of human infections has been performed by the National Reference Laboratory since 1972 through the use of questionnaires sent to hospitalized TBE patients with confirmed laboratory diagnosis.6 These data are shown in Figure 3. Although many of the most affected regions remained constant throughout the observation period, new endemic zones – especially in previously unaffected alpine regions in western Austria – have become established.6 The first TBE case in the federal province of Tyrol was documented in 1984 and in Vorarlberg in 2000. In the subsequent years, certain valleys in both states became sites of infection for a substantial number of human TBE cases.6 In parallel, the incidences in the northeastern part of the country (comprising regions with relatively low altitudes) declined,6 suggesting a change to less favorable conditions for virus circulation in this area. In the traditional core TBE zones of Austria, no evidence has been seen for a shift of infection sites to higher altitudes.6
The causes for establishment of new endemic regions in Austria as well as the decline of TBE in other parts of the country are unknown. Surprisingly, these changes are not paralleled by similar alterations in the incidence of borreliosis, which is transmitted by the same ticks as TBEV but remained relatively constant over time in all parts of Austria.7 These data rule out that the substantial geographical shifts of TBE incidence are only caused by changes in tick abundance or human behavior affecting the risk of tick exposure. The discordant epidemiology of TBE and borreliosis in some parts of Austria rather suggests the existence of yet undefined virus-specific factors that control the circulation of TBEV in its animal reservoir and is independent of general factors controlling the proliferation of ticks.
Overview of TBE in Austria
| Table 1: TBE in Austria | |
|---|---|
| Viral subtypes isolated | European TBEV subtypes5 (and Gerhard Dobler, personal communication; Stephan W. Aberle and Jeremy V. Camp, unpublished results) |
| Reservoir animals | Not known |
| Infected tick species (%) | No information available |
| Dairy product transmission | Small outbreaks3,4 |
| Case definition used by authorities | ECDC |
| Type of reporting | Mandatory for clinically and serologically verified viral meningoencephalitis8 |
| Other TBE-surveillance | No information available |
| Special clinical features | Mild clinical course (febrile illness, meningitis): 36.5%. Severe clinical course (meningoencephalitis, encephalomyelitis, radiculitis): 63.5%. Data of the National reference center for 2023 |
| Licensed vaccines | Encepur Erwachsene, Encepur Kinder (Bavarian Nordic) FSME-IMMUN Erwachsene, FSME-IMMUN Kinder (Pfizer) |
| Vaccination recommendations | General recommendation https://www.sozialministerium.at/Themen/Gesundheit/Impfen/Impfplan-%C3%96sterreich.html |
| Vaccine uptake | ~80%9 |
| National Reference center for TBE | National reference center for human arbovirus infections Center for Virology, Medical University of Vienna Kinderspitalgasse 15, 1090 Vienna, Austria virologie@meduniwien.ac.at |
Figure 1: Incidence of TBE in Austria in total and unvaccinated population, 2010–2023. Update for 2024: 158 reported cases.
Click the image above to enlarge
Orange columns: TBE incidence in the total population
Grey columns: TBE incidence in the unvaccinated population (based on patients with a documented status of ‘no vaccination’). Population data were obtained from the Austrian Statistical Office (“Statistik Austria”, https://www.statistik.at/) and vaccination coverage data from reference.10
| Total | Unvaccinated | |
|---|---|---|
| 2010 | 0.75 | 3.99 |
| 2011 | 1.35 | 7.41 |
| 2012 | 0.62 | 3.09 |
| 2013 | 1.17 | 4.98 |
| 2014 | 0.94 | 5.23 |
| 2015 | 0.82 | 4.48 |
| 2016 | 1.02 | 4.85 |
| 2017 | 1.32 | 5.62 |
| 2018 | 1.74 | 9.13 |
| 2019 | 1.22 | 4.72 |
| 2020 | 2.42 | 8.85 |
| 2021 | 1.43 | 5.14 |
| 2022 | 1.98 | 7.73 |
| 2023 | 1.15 | 4.92 |
Figure 2: Age and gender distribution of TBE in Austria, 2010–2023.

Click the image above to enlarge
| Age Groups | Cases (n) |
|---|---|
| 0-6 | 98 |
| 7-14 | 116 |
| 15-20 | 67 |
| 21-30 | 115 |
| 31-40 | 110 |
| 41-50 | 184 |
| 51-60 | 297 |
| 61-70 | 309 |
| 71-80 | 215 |
| >80 | 62 |
Figure 3: Sites of TBEV infection in Austria, 1972–2023
Click the image above to enlarge
Infection sites were geocoded and processed for spatial mapping by QGIS (https://www.qgis.org/). Spatially close sites were aggregated using a 2 km raster for Austria, and centroids were calculated for each square. These centroids formed the center of the red circles with diameters proportional to the number of documented infection sites within this area. The base map was built using Natural Earth Data [borders, rivers, lakes, cities; http://www.naturalearthdata.com/] and Global Multi-Resolution Topography (GMRT) synthesis data of the Marine Geoscience Data System (MGDS) [topography; 11, http://www.marine-geo.org/tools/GMRTMapTool/].
Contact
Karin Stiasny
karin.stiasny@meduniwien.ac.at
Authors
Karin Stiasny, Simon Raffl, Stephan W. Aberle, Judith H. Aberle
Citation
Stiasny K, Raffl S, Aberle SW, Aberle JH. TBE in Austria. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJS, eds. The TBE Book. 7th ed. Singapore: Global Health Press; 2024. doi:10.33442/26613980_13-1-7
References
- Amato-Gauci A, Zeller H. Tick-borne encephalitis joins the diseases under surveillance in the European Union. Euro Surveill. 2012;17(42):20299.
- Heinz FX, Stiasny K, Holzmann H, Grgic-Vitek M, Kriz B, Essl A, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19(1):69-76. doi:10.3201/eid1901.120458.
- Holzmann H, Aberle SW, Stiasny K, Werner P, Mischak A, Zainer B, et al. Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Emerg Infect Dis. 2009;15(10):1671-3. doi:10.3201/eid1510.090743.
- Mylonaki E, Seiberl M, Jones N, et al. Tick-borne encephalitis virus RNA found in frozen goat’s milk in a family outbreak. Int J Mol Sci. 2022;23(19):11632. doi:10.3390/ijms231911632.
- Ecker M, Allison SL, Meixner T, Heinz FX. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol. 1999;80:179-85. doi:10.1099/0022-1317-80-1-179.
- Heinz FX, Stiasny K, Holzmann H, Kundi M, Sixl W, Wenk M, et al. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Euro Surveill. 2015;20(13). doi:10.2807/1560-7917.es2015.20.13.21077.
- Stiasny K, Santonja I, Holzmann H, et al. The regional decline and rise of tick-borne encephalitis incidence do not correlate with Lyme borreliosis, Austria, 2005 to 2018. Euro Surveill. 2021;26(35):2002108. doi:10.2807/1560-7917.ES.2021.26.35.2002108.
- Bundesministerium für Soziales G, Pflege und Konsumentenschutz. Anzeigenpflichtige Krankheiten in Österreich (gem. Epidemiegesetz, BGBl. Nr. 186/1950 idgF, Tuberkulosegesetz BGBl. Nr. 127/1968, AIDS-Gesetz, BGBl. Nr. 728/1993 idgF, Geschlechtskrankheitengesetz, StGBl. Nr. 152/1945 idgF). In. Anzeigenpflichtige Krankheiten in Österreich. Vol 12023:8.
- Pilz A, Erber W, Schmitt HJ. Vaccine uptake in 20 countries in Europe 2020: Focus on tick-borne encephalitis (TBE). Ticks and Tick-borne Diseases. 2023;14(1):102059. doi:10.1016/j.ttbdis.2022.102059.
- Kunze M, Erber W, Haditsch M. TBE as a matter of public health. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book (6th edition). Global Health Press, Singapore, 2023:365-372. doi:10.33442/26613980_13-4
- Ryan WBF, Carbotte SM, Coplan JO, et al. Global Multi-Resolution Topography synthesis. Geochemistry, Geophysics, Geosystems. 2009;10(3):2008GC002332. doi:10.1029/2008GC002332