Anna Nagy, Ferenc Schneider, Eszter Mezei, András Lakos
E-CDC risk status: endemic
(last edited in May 2025, update for 2024: 34 reported cases)
History and Current Situation
Hungarian scientists were among the pioneers in Europe as the tick-borne encephalitis virus (TBEV) was isolated in 1952, 30 years after the TBEV had been described in Russia (see chapters 3).1 However, most of their observations were published in the Hungarian language, and therefore did not become widely distributed. Between 1981 and 1997, the average annual number of TBE cases reported to authorities was around 300, and as of that year, it decreased to fewer than 20 patients per year (Figures 1, 2). It has been speculated that the decrease is a result of underreporting of TBE, following a change in the reimbursement system for payments related to serologic TBE diagnosis.2-4 However, two main arguments contradict the ‘underreporting hypothesis’:
- During the 5 years before 1997, a total of 1,800,000 FSME vaccine doses were sold by pharmacies (Figure 1), and this convincingly explains the observed reduction of TBE cases (total Hungarian population size is 10 million). Furthermore, after 1997, lethal TBE cases decreased in parallel with decreased incidence. If lower incidences had resulted from underreporting, then lethal cases would not have changed since the etiology of a lethal case is regularly determined by mandatory autopsy and other diagnostic tests.
- The incidence data from the Hungarian military are similar to that of the civilian population: no case has been reported since 2003. ‘Underreporting’5 in this context would be practically impossible. The reporting system for TBE has not changed, and a reduction of cases (most probably due to vaccination) sufficiently explains why the use of TBE serology was subsequently reduced.
Overview of TBE in Hungary
| Table 1: Virus, vector, transmission of TBE in Hungary | |
|---|---|
| Viral subtypes, distribution | TBEV-EU6 |
| Reservoir animals | Apodemus agrarius, Apodemus flavicollis, Microtus arvalis, Myodes glareolus6 Apodemus flavicollis, Apodemus agrarius, Myodes glareolus, Microtus subterraneus7 |
| Infected tick (Fig. 3) | 2/2485=0.08%1 6/8310≈0.07%8 40/51,746≈0.08%; the highest figure was 22/6738≈0.3% in this study9 1/17,500≈0.006%10 5/2196≈0.23%, only with PCR11 3/9616≈0.03%7 |
| Dairy product transmission | Out of the 81 food-borne TBE cases registered between 1992 and 2011, 55.1% were male. Also, 4.4% of the total number of TBE cases were milk-borne. On average, 24.5% of people who drank infected goat milk suffered from clinical symptoms of neurologic infection. Historically, only 2 TBE epidemics in Hungary were caused by cow milk.12 The largest epidemic came from a single goat (of the 75 tested animals) with 25 cases amongst 154 subjects who had consumed contaminated milk.13 In that year (2007), almost half of the total number (30/63) of registered TBE cases were of alimentary origin. |
| Table 2: TBE reporting and vaccine prevention in Hungary | |
|---|---|
| Mandatory TBE reporting | Every physician who establishes a diagnosis of TBE must report it. Practically, these are hospital-based specialists for infectious diseases, pediatricians, internists, and neurologists. Case definition: clinical symptoms of central nervous infection + presence of TBE immunoglobulin M (IgM) antibodies in serum and cerebrospinal fluid (CSF) OR TBEV-specific IgM in CSF OR isolation of infectious virus from clinical samples OR detection of TBEV RNA in clinical samples OR seroconversion and/or 4-fold specific IgG increase in a sample pair.14 |
| Other TBE surveillance | No |
| Special clinical features | 1. In one study, 21% of retrospectively collected patient cases were agrarian, 16% forestry workers.8 2. Other work has shown 12% to 16% of patients with TBE were forestry workers.9,10 3. Similarly, another report found 10.4% of 5196 cases were forestry, 11% other agrarian workers.15 4. Also, 2% of the 1,670 forestry workers screened for Lyme borreliosis went through TBE (Lakos, unpublished data). 5. 65% of hospitalized patients could recall a biphasic course of their TBE.16 In the same department of the Central Hospital for Infectious Diseases, during the years 1976-1980 (n=100), 27 patients showed paresis, 2 died. In 1987-1991 (n=93), only 5 patients had paresis, none of them died.17 From 1985 to 2008, the death rate from TBE in Hungary was 29/3987 (0.73%).18 However, in an earlier period from 1977 to 1996, the fatality rate was higher – 43/5196 (0.83%). Most of the fatal cases were male (85%), while the proportion of male patients in the total TBE population was 70%.15 |
| Available vaccines | FSME Immune Inject is a vaccine available for public use since 1992; another vaccine, Encepur, launched in 1995. Previously, between 1977 and 1990, some 150,000 doses were distributed for the at-risk population. It has to be mentioned, that TBE vaccination in Austria at the same time showed a field effectiveness 79.4%-100% after the second dose and 97.3%-100% after the third dose26) From 1990 to 2017, 6 million doses were sold. (The Hungarian population is 10 million). |
| Vaccination recommendations and reimbursement | When FSME Immune Inject was first available in Hungary in the early 1990s, the reimbursement rate was 95%; the pharmacy price was 59 HUF (€ 0.20). After a gradual decrease, the reimbursement was cancelled for the FSME and Encepur vaccines in 2008 and 2012, respectively. The present price is around 13,000 HUF (€ 40). For occupationally exposed workers, vaccination has been mandatory at the employers’ expense since 1999.20 |
| Vaccine uptake by age group/risk group/general population | Not available |
| Name, address/website of TBE National Reference Center | National Center for Public Health and Pharmacy, National Reference Laboratory for Viral Zoonoses, Budapest, Hungary [https://www.nnk.gov.hu/]. |
Figure 1: Gender distribution of TBE cases and the sold number of doses of TBE vaccines. Update for 2024: 34 reported cases.
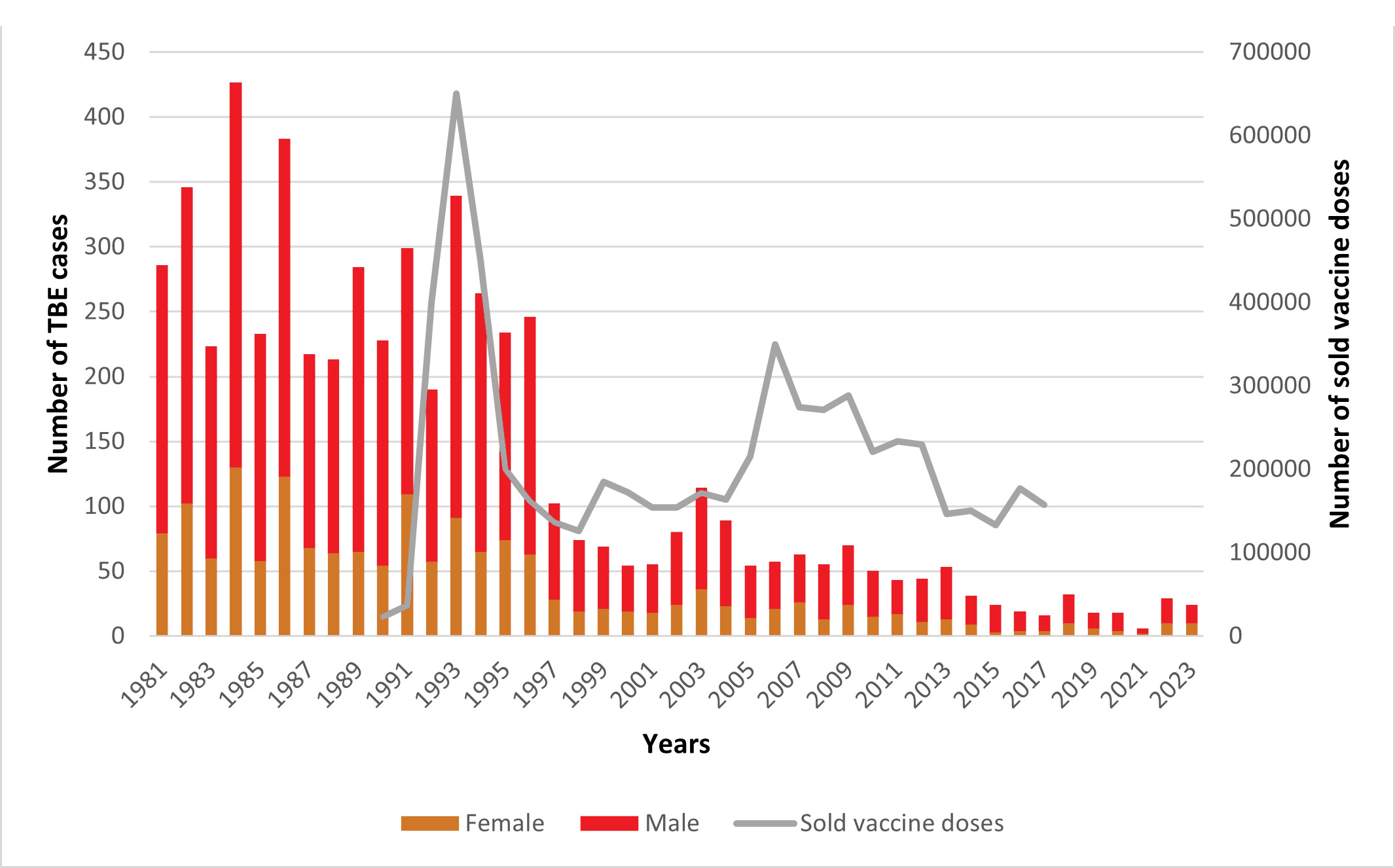
The data of TBE cases in this graph originated from the National Reference Laboratory for Viral Zoonoses and from the Department of Epidemiological and Vaccination Surveillance of the National Center for Public Health and Pharmacy. The data for 1998 is missing, an estimation is plotted in the graph. No reliable information on the number of vaccine doses sold in 1995 could be found; estimated information was used. (The number of vaccine doses sold is not available from 2018.)
Figure 2: Burden of TBE in Hungary from 1981 to 202324,25. Age distribution and the number of the tested patients
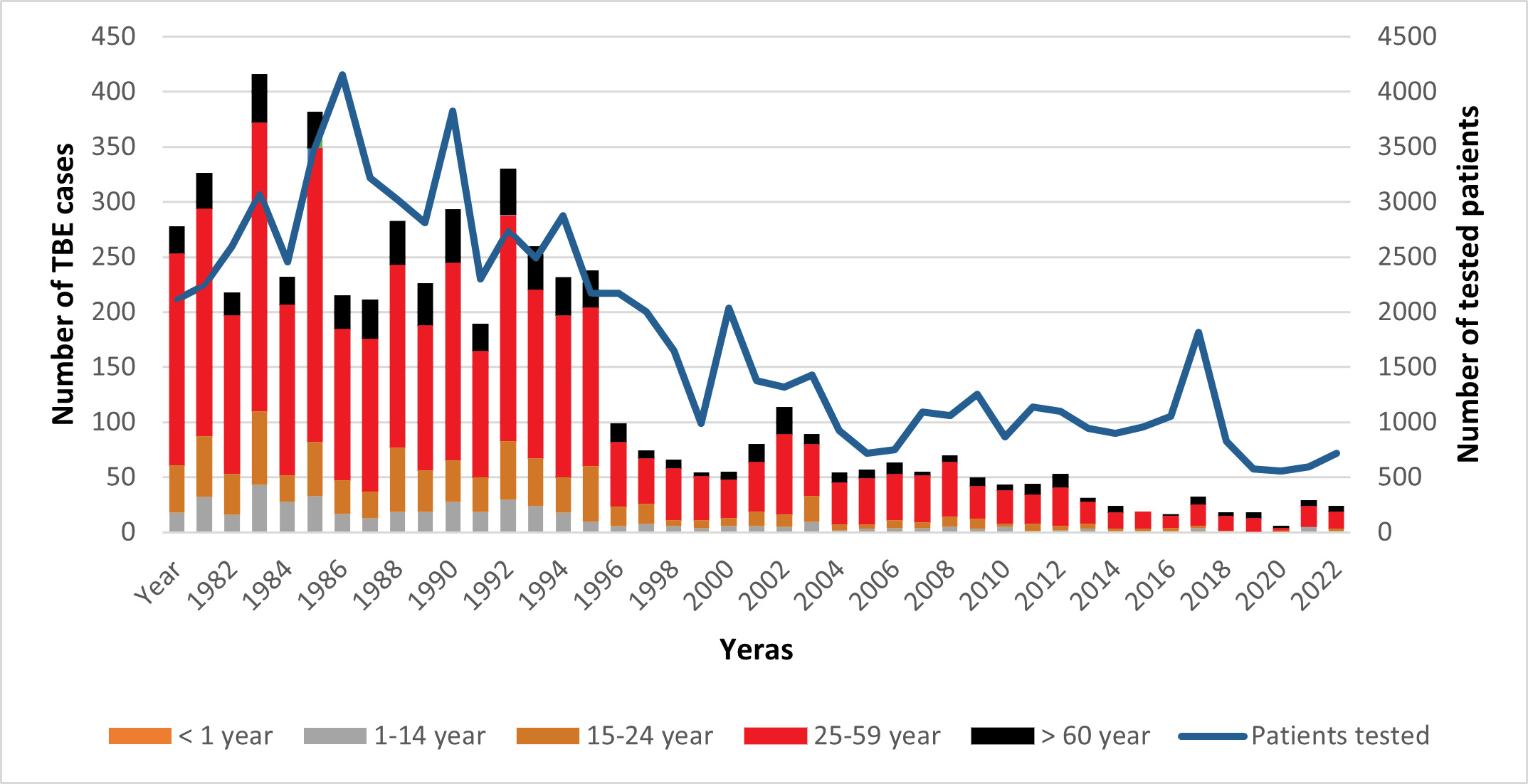
The data of TBE cases in this graph originated from the National Reference Laboratory for Viral Zoonoses and from the Department of Epidemiological and Vaccination Surveillance of the National Center for Public Health and Pharmacy. The number of TBE cases decreased dramatically after a mass vaccination campaign from 1992 to 1995. The Hungarian population is approximately 10 million, so the incidence for 100 cases is 1/100,000. A West Nile virus epidemic resulted in 225 WNV infections in 2018 (https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2019.24.28.1900038). That was the reason for the striking elevation of the requested TBE serological tests (number of tested patients). The elevated number of tests coincided with the elevated number of verified TBE cases.
| Year | Female | Male | <1 year | 1–14 years | 15–24 years | 25–59 years | >60 years | Unknown age | Total TBE cases | Sold vaccine doses | Samples tested (IgG) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1981 | 79 | 207 | 0 | 18 | 43 | 192 | 25 | 8 | 286 | N/A | 2113 |
| 1982 | 102 | 244 | 0 | 32 | 55 | 207 | 32 | 20 | 346 | N/A | 2241 |
| 1983 | 60 | 163 | 0 | 16 | 37 | 144 | 21 | 5 | 223 | N/A | 2595 |
| 1984 | 130 | 297 | 0 | 43 | 67 | 262 | 44 | 11 | 427 | N/A | 3074 |
| 1985 | 58 | 175 | 0 | 28 | 24 | 155 | 25 | 1 | 233 | N/A | 2456 |
| 1986 | 123 | 260 | 0 | 33 | 49 | 267 | 33 | 1 | 383 | N/A | 3486 |
| 1987 | 68 | 149 | 0 | 17 | 30 | 138 | 30 | 2 | 217 | N/A | 4157 |
| 1988 | 64 | 149 | 0 | 13 | 24 | 139 | 35 | 2 | 213 | N/A | 3215 |
| 1989 | 65 | 219 | 0 | 19 | 58 | 166 | 39 | 2 | 284 | N/A | 3016 |
| 1990 | 54 | 174 | 0 | 19 | 37 | 132 | 38 | 2 | 228 | 23251 | 2809 |
| 1991 | 109 | 190 | 0 | 28 | 37 | 180 | 48 | 6 | 299 | 36,720 | 3823 |
| 1992 | 57 | 133 | 0 | 19 | 31 | 115 | 24 | 1 | 190 | 400,000 | 2301 |
| 1993 | 91 | 248 | 0 | 30 | 53 | 205 | 42 | 9 | 339 | 650,000 | 2737 |
| 1994 | 65 | 199 | 0 | 24 | 43 | 153 | 40 | 4 | 264 | 450,000 | 2488 |
| 1995 | 74 | 160 | 0 | 18 | 32 | 147 | 34 | 3 | 234 | 200,000 | 2875 |
| 1996 | 63 | 183 | 0 | 10 | 50 | 144 | 34 | 8 | 246 | 161,717 | 2168 |
| 1997 | 28 | 74 | 0 | 6 | 17 | 59 | 17 | 3 | 102 | 136,394 | 2168 |
| 1998 | 19 | 55 | 0 | 8 | 18 | 41 | 7 | 0 | 74 | 125,843 | 2000 |
| 1999 | 21 | 48 | 0 | 6 | 5 | 47 | 8 | 3 | 69 | 184,555 | 1649 |
| 2000 | 19 | 35 | 0 | 4 | 7 | 40 | 3 | 0 | 54 | 172,615 | 988 |
| 2001 | 18 | 37 | 0 | 6 | 7 | 35 | 7 | 0 | 55 | 153,941 | 2036 |
| 2002 | 24 | 56 | 0 | 6 | 13 | 45 | 16 | 0 | 80 | 154,165 | 1379 |
| 2003 | 36 | 78 | 0 | 5 | 11 | 73 | 25 | 0 | 114 | 171,151 | 1315 |
| 2004 | 23 | 66 | 0 | 10 | 23 | 47 | 9 | 0 | 89 | 163,347 | 1428 |
| 2005 | 14 | 40 | 0 | 2 | 5 | 38 | 9 | 0 | 54 | 215,238 | 927 |
| 2006 | 21 | 36 | 0 | 3 | 4 | 42 | 8 | 0 | 57 | 349,206 | 467 |
| 2007 | 26 | 37 | 0 | 4 | 7 | 42 | 10 | 0 | 63 | 274,396 | 750 |
| 2008 | 13 | 42 | 0 | 4 | 5 | 43 | 3 | 0 | 55 | 271,092 | 1636 |
| 2009 | 24 | 46 | 0 | 5 | 9 | 50 | 6 | 0 | 70 | 288,629 | 1527 |
| 2010 | 15 | 35 | 0 | 3 | 9 | 30 | 8 | 0 | 50 | 221,095 | 1154 |
| 2011 | 17 | 26 | 0 | 5 | 3 | 30 | 5 | 0 | 43 | 233,579 | 1003 |
| 2012 | 11 | 33 | 0 | 1 | 7 | 26 | 10 | 0 | 44 | 229,794 | 1095 |
| 2013 | 13 | 40 | 0 | 2 | 4 | 35 | 12 | 0 | 53 | 146,518 | 1099 |
| 2014 | 9 | 22 | 0 | 3 | 5 | 20 | 3 | 0 | 31 | 150,507 | 840 |
| 2015 | 3 | 21 | 0 | 1 | 2 | 15 | 6 | 0 | 24 | 132,878 | 855 |
| 2016 | 4 | 15 | 0 | 1 | 2 | 16 | 0 | 0 | 19 | 177,064 | 958 |
| 2017 | 4 | 12 | 0 | 1 | 3 | 11 | 1 | 0 | 16 | 157,687 | 1050 |
| 2018 | 10 | 22 | 0 | 4 | 2 | 19 | 7 | 0 | 32 | N/A | 1814 |
| 2019 | 6 | 12 | 0 | 0 | 1 | 14 | 3 | 0 | 18 | N/A | 830 |
| 2020 | 4 | 14 | 0 | 0 | 0 | 13 | 5 | 0 | 18 | N/A | 578 |
| 2021 | 2 | 4 | 0 | 0 | 1 | 3 | 2 | 0 | 6 | N/A | 553 |
| 2022 | 10 | 19 | 0 | 5 | 0 | 19 | 5 | 0 | 29 | N/A | 597 |
| 2023 | 10 | 14 | 0 | 1 | 2 | 16 | 5 | 0 | 24 | N/A | 719 |
Figure 3: TBEV-isolation and TBE cases in Hungary

Map of Hungary showing human TBE incidence (ECDC, 2017).22 TBEV isolation sites are marked by circle (Fornosi, 1954),1 six-pointed stars (Molnár, 1979),23 five-pointed stars (Gerzsenyi, 1980 and 1985),9,10 four-pointed star (Pintér, 2013),11 and three-pointed star (Zöldi, 2015).7 A map with more detailed incidence data21 can be downloaded from https://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20128.
Contact
Anna Nagy
nagy.anna@nngyk.gov.hu
Authors
Anna Nagy, Ferenc Schneider, Eszter Mezei, András Lakos
Citation
Nagy A, Schneider F, Mezei E, Lakos A. TBE in Hungary. Chapter 13. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ, eds. The TBE Book. 7th ed. Singapore: Global Health Press; 2024. doi:10.33442/26613980_13-14-7
References
- Fornosi F, Molnár E. [Isolation of encephalomyelitis virus from ticks. I. Isolation of the virus and its properties]. Orv Hetil. 1954;95(6):144-149.
- Randolph SE; EDEN-TBD sub-project team. Human activities predominate in determining changing incidence of tick-borne encephalitis in Europe. Euro Surveill. 2010;15(27):24-31. Published 2010 Jul 8. doi:10.2807/ese.15.27.19606-en
- Petri E, Gniel D, Zent O. Tick-borne encephalitis (TBE) trends in epidemiology and current and future management. Travel Med Infect Dis. 2010;8(4):233-245. doi:10.1016/j.tmaid.2010.08.001
- World Health Organisation. Vector-borne human infections in Europe. distribution and burden on public health. 2004. Accessed 16 January, 2011. https://www.euro.who.int/document/e82481.pdf.
- Lakos A, Rókusz L. [Epidemiology of tick-borne encephalitis in Hungary – significance of the vaccination]. Háziorvos Továbbképző Szemle. 16:38-40.
- Pintér R, Madai M, Horváth G, et al. Molecular detection and phylogenetic analysis of tick-borne encephalitis virus in rodents captured in the transdanubian region of Hungary. Vector Borne Zoonotic Dis. 2014;14(8):621-624. doi:10.1089/vbz.2013.1479
- Zöldi V, Papp T, Rigó K, Farkas J, Egyed L. A 4-year study of a natural tick-borne encephalitis virus focus in Hungary, 2010-2013. Ecohealth. 2015;12(1):174-182. doi:10.1007/s10393-014-0969-0
- Kubinyi L, Molnár E, Szertich A, Pethő I. [Tick encephalitis in Zala and Győr-Sopron counties]. Orv Hetil. 1971;112:2931-5.
- Gerzsenyi K. [Tick-borne meningoencephalitis investigations in the Transdanubian and the Budapest regions and the prevention]. Forest Research Institute. 1980 p23.
- Gerzsenyi K. [Foci of tick-borne encephalitis virus in Central and Eastern Hungary]. Forest Research Institute, 1985 p 25.
- Pintér R, Madai M, Vadkerti E, et al. Identification of tick-borne encephalitis virus in ticks collected in Southeastern Hungary. Ticks Tick Borne Dis. 2013;4:427-31. doi:10.1016/j.ttbdis.2013.04.008
- Zöldi V, Ferenczi E, Egyed L. [Milk-transmitted tick-borne encephalitis epidemics in Hungary]. Magyar Állatorvosok Lapja. 2013;135:48-56.
- Balogh Z, Ferenczi E, Szeles K, et al. Tick-borne encephalitis outbreak in Hungary due to consumption of raw goat milk. J Virol Methods. 2010;163:481-5. doi:10.1016/j.jviromet.2009.10.003
- Kollaritsch H, Chmelík V, Dontsenko I, et al. The current perspective on tick-borne encephalitis awareness and prevention in six Central and Eastern European countries: report from a meeting of experts convened to discuss TBE in their region. Vaccine. 2011;29:4556-64. doi:10.1016/j.vaccine.2011.04.061
- Lontai I, Straub I. Tick-borne encephalitis and its prevention in Hungary. Med Pregl. 1998;51 Suppl 1:21-3.
- Schneider F, Pergel R. [Tick-borne encephalitis in Vas county between 1988-1998]. Vasi Szemle.2000;54:827-38.
- Lakos A, Ferenczi E, Ferencz A, Tóth E. Tick-borne encephalitis. Parasit Hung. 1997;30:5-16.
- Zöldi V, Erdős Gy, Szlobodnyik J. [2nd Guideline on tick control and prevention]. EPINFO. 2009;16:Suppl 3 p 61.
- Ferenczi E, Molnár E. Tick-borne encephalitis in the last ten years. Ellipse. 29:458-9.
- Lakos A. [Tick-borne encephalitis, benefit of the vaccine.] Háziorvosi Szemle. 2012;17:36-7.
- Caini S, Szomor K, Ferenczi E, et al. Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011 [published correction appears in Euro Surveill. 2012;17(31): pii/20236]. Euro Surveill. 2012;17(12):20128. Published 2012 Mar 22.
- ECDC.situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. Accessed January 5, 2017. https://ecdc.europa.eu/en/publications/Publications/TBE-in-EU-EFTA.pdf.
- Molnár E. Location of the tick-borne encephalitis and other arboviruses and their burden in Hungary. DSc thesis.1979, p 193. Cited in: Zöldi V, Erdős Gy, Szlobodnyik J.nd Guideline on tick control and prevention(in Hungarian). EPINFO. 2009;16:Suppl 3 p 61.
- OEK (National Centre for Epidemiology), 2016. Data on file.
- IMSHealth Hungary, 2010. Data on file
- Kunz C. Tick-borne encephalitis in Europe. Acta Leiden. 1992;60(2):1-14.