Valentina Tagliapietra, Martina Del Manso, Flavia Riccardo and Anna Teresa Palamara
E-CDC risk status: endemic
(last edited in May 2025, update for 2024: 48 reported cases)
History and current situation
Italy is considered a low-incidence country for tick-borne encephalitis (TBE) in Europe.1 Areas at higher risk for TBE within Italy are geographically clustered in the forested and mountainous regions and provinces of the northeastern part of the country, as suggested by TBE case series published over the last decade. 2–4 A national enhanced surveillance system for TBE has been established since 2017.5 Before this, information on the occurrence of TBE cases at the national level in Italy was lacking. Both incidence rates and the geographical distribution of the disease were mostly inferred from endemic areas where surveillance was already in place, and from ad hoc studies and international literature. TBE has been recorded in Italy since 1967, with foci of infections in the northeast (Trento, Belluno and Gorizia) and central (Florence and Latina) provinces.6–9 TBE presence in central Italy has not been confirmed by further studies on ticks and serosurveys conducted afterwards,10,11 nor by human cases, posing concerns about possible misdiagnosis.
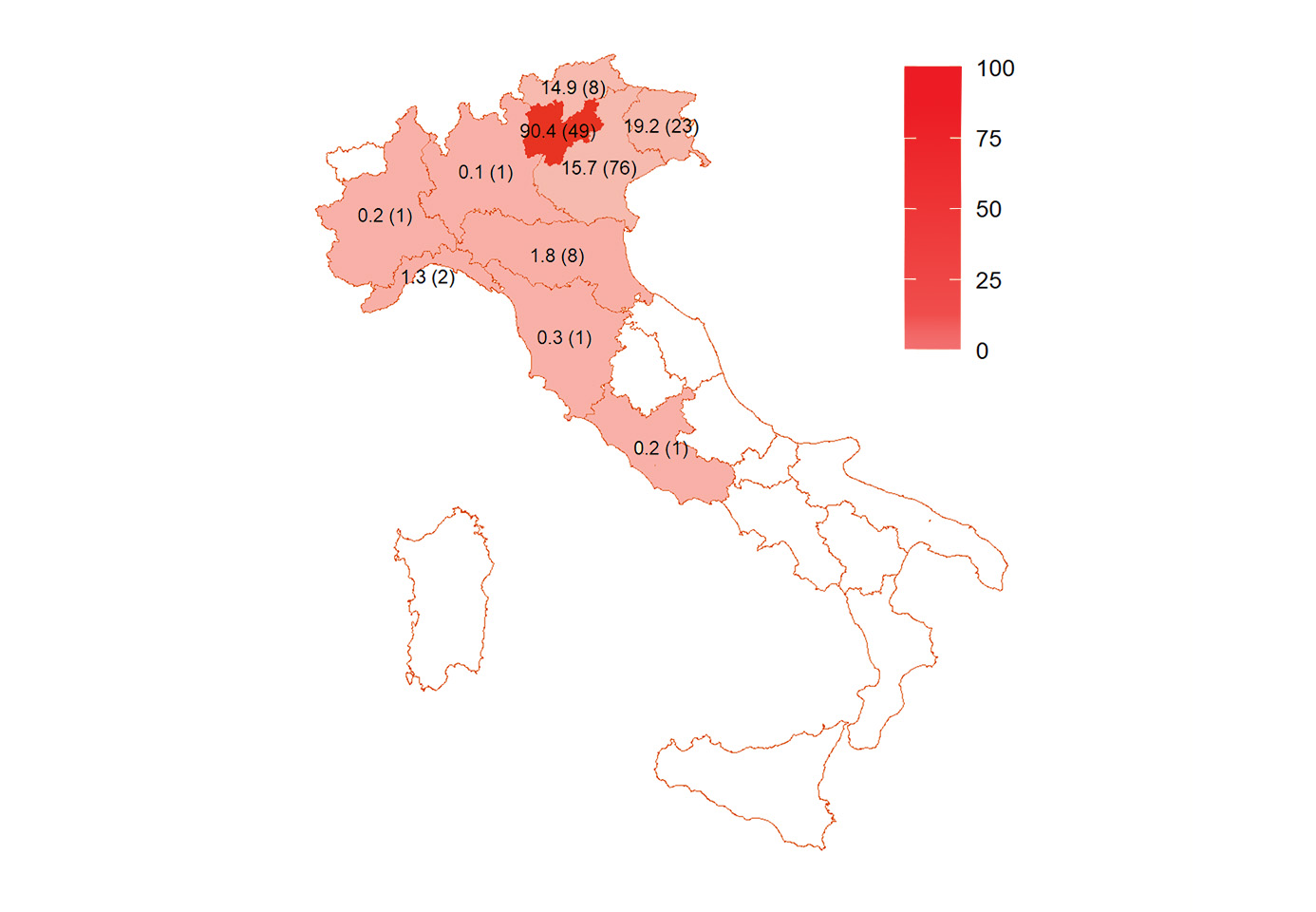
Serological investigations of people at risk, such as forestry rangers, hunters, hikers and forest products collectors, have been performed in order to get information on human exposure to TBE virus (TBEV). Circulation in the pre-alpine and alpine regions reported partially NT-confirmed seroprevalence values of 0.6%, 1.07% and 3.2% in Friuli-Venezia Giulia,12 Trento province13 and Turin province,14 respectively. Interestingly, Turin province has never reported TBE human cases, so far. A retrospective study conducted in 2015 in the northeast regions using the ECDC case definition of TBE,3 allowed the identification of 367 cases (0.38 per 100,000 inhabitants) during the period from 2000 to 2013.3 TBE cases were mainly males (70%) and the majority of them were between 30 and 70 years of age (see also Figure 2). A significant increase in the annual incidence rate (IR) was observed during the study period, from 0.18 per 100,000 in the year 2000 up to 0.59 per 100,000 in 2013 (95% confidence interval [CI]: 1.02–1.08, P>0.01).3 The majority of TBE cases occurred between April and October, consistent with the seasonal activity of ticks. According to this study, the risk of TBE is associated with altitude, with the highest values found for municipalities between 400 and 600 m a.s.l., and the IR falling along with municipality altitude decrease or increase.3 In 2022, TBE showed a record in the number of cases and mortality rates, with 72 cases, mainly from four northeastern Italian regions and provinces15: Trento (18 cases), Friuli-Venezia Giulia (12 cases) and Veneto (37 cases), and sporadically from other locations i.e. Emilia Romagna (2 cases), Liguria (2 cases) and Lazio (1 case) (Fig. 3) and 3 fatal events, resulting in an exceptionally high mortality rate of 4.17%.
In its natural enzootic cycle, TBEV transmission involves ixodid ticks, mainly belonging to the genus Ixodes, and the small mammal hosts (rodents and insectivores) which support both ticks population and TBEV circulation. The link between tree masting, rodent population dynamics, density of nymphal ticks and eventually the incidence of tick-borne diseases in humans, has been investigated in several studies highlighting the expected two-year lag between a masting event and the increase in (infected) nymphs.16,17 In this context, a long-term study conducted in the Province of Trento positively correlated pollen data and TBE incidence in humans,18 therefore offering to public health agencies a potential early warning tool that might be used to plan preventive measures two years in advance. Of note is the fact that a huge mast event involving two important forest species (Fagus sylvatica and Picea abies) was recorded in 2020 and that the peak in the number of TBE cases happened in 2022.
In particular, the province of Trento showed a sharp increase in TBE incidence since 2012, despite vaccination efforts. To assess the current risk of infection in the provincial territory, an integrated One-Health research approach was applied, combining the analysis of the distribution of human cases, the study of seroprevalence in sentinel hosts (goats) and the direct screening of questing ticks.19 A total of 1.56% of goats resulted positive for specific antibodies for TBEV. Sampling of ticks was concentrated in areas where TBEV circulation was observed both in seropositive goats or in humans, resulting in a prevalence of 0.17%. In particular these results revealed an increased prevalence of TBEV in ticks and the emergence of new active TBE foci which are located northward and at higher altitude (1.109 m a.s.l.) compared to previous investigations. None of the areas with seropositive goats was confirmed by TBEV detection in ticks and recent human cases, but this aspect needs further confirmation.
Since the 1990s, rising cervid population numbers and changes in forest structure in the northeastern regions and provinces of Italy were observed in conjunction with an increase in TBE incidence,20 but this relationship is not always positive and at a threshold density level of ungulates, TBEV prevalence decreases.21 Transmission of TBEV from infected nymphs to co-feeding uninfected ticks on rodents is considered the most efficient route for the amplification of this virus, therefore, studies regarding the ecological and abiotic conditions affecting tick feeding dynamics are important. Recently a long-term longitudinal field study highlighted that the autumnal cooling rate and the presence of roe deer and mice are crucial ecological drivers for co-feeding transmission which in turn is reflected in the maintenance of a TBEV hotspot.22 The animal community composition and abundance are known to affect transmission of tick-borne diseases, suggesting that in highly diverse habitats TBE risk decreases. Using habitat richness as a proxy for vertebrate host diversity, high TBE risk corresponded to areas with intermediate richness. In endemic areas, such as those located in northeast Italy, TBE risk is higher probably because it features habitat types that are generally suitable for both ticks and hosts presence.23
Vaccination for TBE is currently recommended in Italy among residents and occupationally exposed groups, living in rural endemic areas, but its impact on disease occurrence in the affected communities is not yet evaluated.24 In the Friuli-Venezia Giulia region since 2013 and in the Autonomous Provinces of Trento and Bolzano since 2018, TBE vaccine is offered free of charge to the resident population.
In conclusion, the incidence of TBE in Italy is relatively low and the risk appears to be geographically restricted to the pre-alpine and alpine regions of the country. However, recent increase and spread in the number of cases (see Figure 3), pose concerns regarding the importance of disentangling the complex factors that are involved in the spread and maintenance of TBEV in an endemic focus and the early-warning predictors that should be identified.
Overview of TBE in Italy
| Table 1: TBE in Italy | |
|---|---|
| Viral subtypes isolated | European TBEV subtype19 |
| Reservoir animals | Rodents, ticks |
| Infected tick species (%) | 0.17% (Trento Province)19; 2.1% (Belluno province)25 |
| Dairy product transmission | Case definition: Clinical criteria are any symptoms of inflammation of the CNS (for example, meningitis, meningo-encephalitis, encephalomyelitis, encephaloradiculitis). A TBE case is confirmed by at least one of the following five laboratory criteria: TBE specific IgM AND IgG antibodies in blood; TBE specific IgM antibodies in CSF; seroconversion or four-fold increase of TBE-specific antibodies in paired serum samples; detection of TBE viral nucleic acid in a clinical specimen; isolation of TBE virus from clinical specimen |
| Type of reporting | Reported by Department of Infectious Diseases, National Institute of Health, Italy in collaboration with all the Infectious Diseases Units and Public Health Districts. Surveillance has been enhanced at the national level since 2017 and web-based from 2020. Presumed place of exposure and date of tick bite are recorded |
| Other TBE-surveillance | Ticks, rodents and sentinel animals screening |
| Special clinical features | Biphasic disease is not reported |
| Licensed vaccines | TICOVAC 0.5 mL and 0.25 mL (for pediatric use) (Pfizer Srl) |
| Vaccination recommendations | Vaccine is free of charge for residents in the Friuli-Venezia Giulia and Trentino-Alto Adige regions |
| Vaccine uptake | Recommended for those who live, frequent or work in the woods or in rural areas i.e. hikers/trekkers, foragers, agricultural, forest or lumber workers |
| National Reference center for TBE | Prof.ssa Anna Teresa Palamara Dipartimento Malattie Infettive Istituto Superiore di Sanità Viale Regina Elena, 299 00161 Roma, Italia https://www.iss.it |
Figure 1: Reported human cases and incidence of TBE, Italy, 2000–2023. Update for 2024: 48 reported cases.
Data on vaccine uptake not available
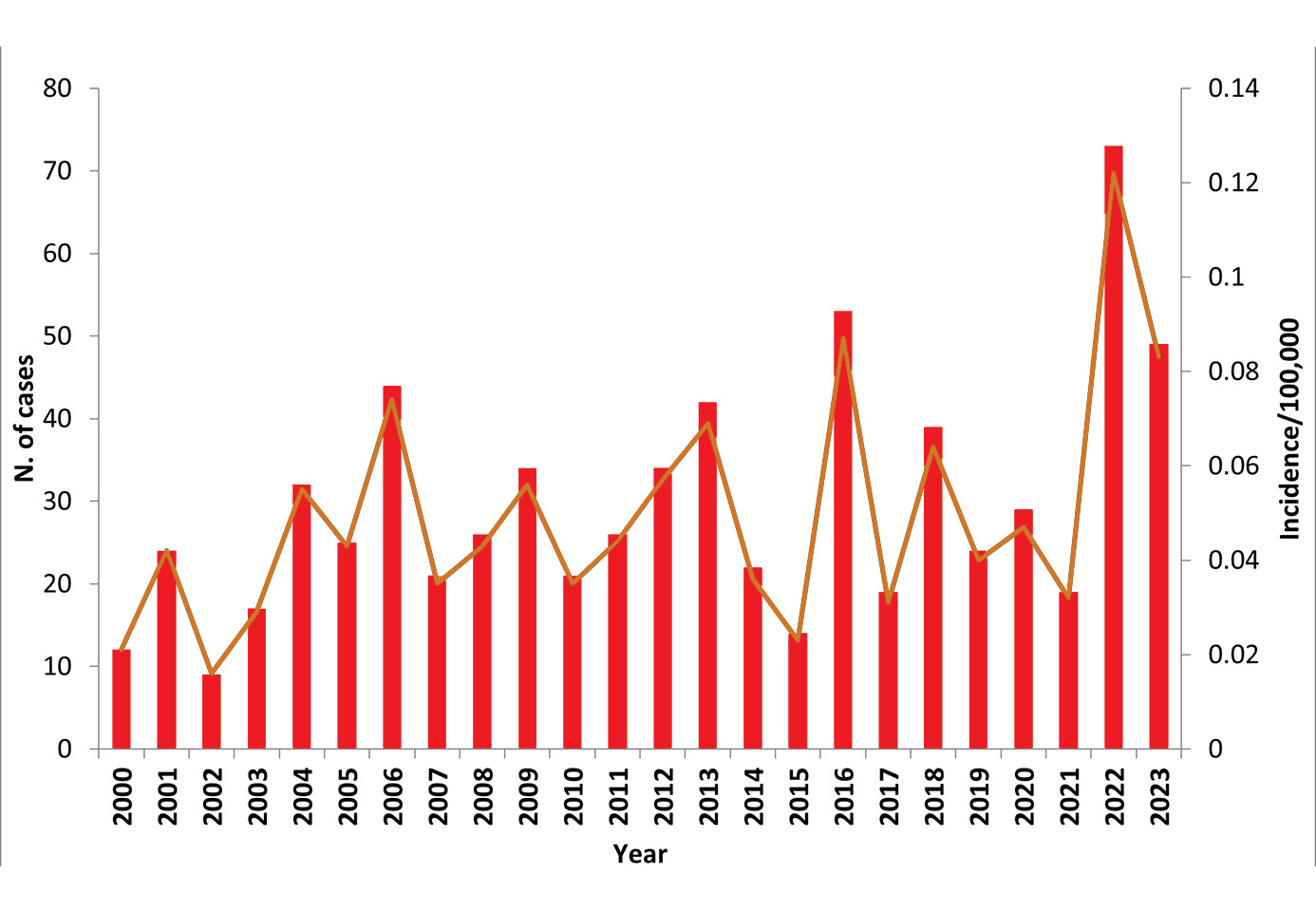
Source data:
| Year | Number of Cases | Incidence / 105 | Vaccination rate (%) |
|---|---|---|---|
| 2000 | 12 | 0.021 | |
| 2001 | 24 | 0.042 | |
| 2002 | 9 | 0.016 | |
| 2003 | 17 | 0.029 | |
| 2004 | 32 | 0.055 | |
| 2005 | 25 | 0.043 | |
| 2006 | 44 | 0.074 | 0.11 |
| 2007 | 21 | 0.035 | 0.11 |
| 2008 | 26 | 0.043 | 0.11 |
| 2009 | 34 | 0.056 | 0.14 |
| 2010 | 21 | 0.035 | 0.13 |
| 2011 | 26 | 0.044 | 0.16 |
| 2012 | 34 | 0.057 | 0.10 |
| 2013 | 42 | 0.069 | 0.18 |
| 2014 | 22 | 0.036 | 0.15 |
| 2015 | 14 | 0.023 | |
| 2016 | 53 | 0.087 | |
| 2017* | 19 | 0,031 | |
| 2018* | 39 | 0,064 | |
| 2019* | 24 | 0,040 | |
| 2020* | 29 | 0,047 | |
| 2021* | 19 | 0,032 | |
| 2022* | 73 | 0,122 | |
| 2023* | 49 | 0.083 | |
| 2024* | 48 |
Figure 2: Age and gender distribution of reported human cases of neuro-invasive laboratory confirmed TBEV infections, Italy, 2020-2023

| Age group (years) | Males | Females | All |
|---|---|---|---|
| 0-9 | 2 | 0 | 2 |
| 10-19 | 8 | 6 | 14 |
| 20-29 | 4 | 5 | 9 |
| 30-39 | 11 | 1 | 12 |
| 40-49 | 13 | 5 | 18 |
| 50-59 | 26 | 18 | 44 |
| 60-69 | 26 | 10 | 36 |
| >70 | 24 | 11 | 35 |
Figure 3: Distribution (4-year incidence/100,000 and number of cases in 4 years (2020-2023)) of neuro-invasive laboratory confirmed TBE per region/autonomous province (incidence based on each region / province population size) of Italy
Acknowledgments
The authors thank Elisa Di Maggio for her support in data collection and analysis.
Contact
Valentina Tagliapietra
valentina.tagliapietra@fmach.it
Authors
Valentina Tagliapietra, Martina Del Manso, Flavia Riccardo and Anna Teresa Palamara
Citation
Tagliapietra V, Del Manso M, Riccardo F, Palmara A.T. TBE in Italy. Chapter 13. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ, eds. The TBE Book. 7th ed. Singapore: Global Health Press; 2024.doi:10.33442/26613980_13-15-7
References
- Dagostin F, Tagliapietra V, Marini G, et al. Ecological and environmental factors affecting the risk of tick-borne encephalitis in Europe, 2017 to 2021. Euro Surveill. 2023;28(42). doi:10.2807/1560-7917.ES.2023.28.42.2300121
- Beltrame A, Ruscio M, Cruciatti B, et al. Tickborne encephalitis virus, northeastern Italy. Emerg Infect Dis. 2006;12(10):1617-9. doi:10.3201/eid1210.060395
- Rezza G, Farchi F, Pezzotti P, et al. Tick-borne encephalitis in north-east Italy: a 14-year retrospective study, January 2000 to December 2013. Euro Surveill. 2015;20(40):1560-7917. doi: 10.2807/1560-7917.ES.2015.20.40.30034
- Regione Autonoma Friuli Venezia Giulia. L’encefalite da zecca (TBE) in Friuli Venezia Giulia. Accessed March 1, 2024. https://www.regione.fvg.it/rafvg/export/sites/default/RAFVG/salute-sociale/zecche/allegati/TBE_FVG.pdf.
- Ministero della salute. Piano nazionale di sorveglianza e risposta all’encefalite virale da zecche e altre arbovirosi. Accessed March 1, 2024. https://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=3399.
- Amaducci L, Arnetoli G, Inzitari D, Balducci M, Verani P, Lopes M. Tick-borne encephalitis (TBE) in Italy: report of the first clinical case. Riv Patol Nerv Ment. 1976;2(97):77-80.
- Paci P, Leoncini F, Mazzotta F, et al. Meningoencefaliti da zecche (TBE) in Italia. Ann Sclavo. 1980;22(3):404-16.
- Ciufolini M, Verani P, Nicoletti L, et al. Recent advances in the eco‐epidemiology of tick borne encephalitis in Italy. Alpe Adria Microbiology Journal. 1999;8:81-83.
- Verani P, Ciufolini MG, Nicoletti L. Arbovirus surveillance in Italy. Parassitologia. 1995;37:2-3.
- Tomao P, Ciceroni L, D’Ovidio MC, et al. Prevalence and incidence of antibodies to Borrelia burgdorferi and to tick-borne encephalitis virus in agricultural and forestry workers from Tuscany, Italy. Eur J Clin Microbiol Infect Dis. 2005;24(7):457-463. doi:10.1007/s10096-005-1348-0
- Di Renzi S, Martini A, Binazzi A, et al. Risk of acquiring tick-borne infections in forestry workers from Lazio, Italy. Eur J Clin Microbiol Infect Dis. 2010;29(12):1579-1581. doi:10.1007/s10096-010-1028-6
- Cinco M, Barbone F, Ciufolini MG, et al. Seroprevalence of tick-borne infections in forestry rangers from northeastern Italy. Clin Microbiol Infect. 2004;10(12):1056-1061. doi:10.1111/j.1469-0691.2004.01026.x
- Cristofolini A, Bassetti D, Schallenberg G. Zoonoses transmitted by ticks in forest workers (tick-borne encephalitis and Lyme borreliosis): preliminary results. Med Lav. 1993;84(5):394-402.
- Tomassone L, Berriatua E, De Sousa R, et al. Neglected vector-borne zoonoses in Europe: Into the wild. Vet Parasitol. 2018;15(251):17-26. doi:10.1016/j.vetpar.2017.12.018
- Del MM, Di ME, Perego G, et al. Arbovirosi in Italia-2022. Istituto Superiore di Sanità. Accessed Mar 1, 2024. https://www.epicentro.iss.it/arbovirosi/dashboard
- Brugger K, Walter M, Chitimia-Dobler L, Dobler G, Rubel F. Forecasting next season’s Ixodes ricinus nymphal density: the example of southern Germany 2018. Exp Appl Acarol. 2018;75:281-288. doi:10.1007/s10493-018-0267-6
- Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. Climate, deer, rodents and acorns as determinants of variation in lyme-disease risk. PLoS Biol. 2006;4(6):e145. doi:10.1371/journal.pbio.0040145
- Marini G, Tagliapietra V, Cristofolini F, et al. Correlation between airborne pollen data and the risk of tick-borne encephalitis in northern Italy. Sci Rep. 2023;13(1):1-8. Published 2023 May 22. doi:10.1038/s41598-023-35478-w
- Alfano N, Tagliapietra V, Rosso F, Ziegler U, Rizzoli A. Tick-borne encephalitis foci in northeast Italy revealed by combined virus detection in ticks, serosurvey on goats and human cases. Emerg Microbes Infect. 2020;9(1):474-84. doi:10.1080/22221751.2020.1730246
- Rizzoli A, Hauffe HC, Tagliapietra V, Neteler M, Rosà R. Forest structure and roe deer abundance predict Tick-borne encephalitis risk in Italy. PLoS One. 2009;4(2). doi:10.1371/journal.pone.0004336
- Cagnacci F, Bolzoni L, Rosà R, et al. Effects of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. I: Empirical assessment. Int J Parasitol. 2012;42(4). doi:10.1016/j.ijpara.2012.02.012
- Rosà R, Tagliapietra V, Manica M, et al. Changes in host densities and co-feeding pattern efficiently predict tick-borne encephalitis hazard in an endemic focus in northern Italy. Int J Parasitol. 2019;49(10):779-787. doi:10.1016/j.ijpara.2019.05.006
- Dagostin F, Tagliapietra V, Marini G, et al. High habitat richness reduces the risk of tick-borne encephalitis in Europe: A multi-scale study. One Heal. 2023;18:100669. Published 2023 Dec 30. doi:10.1016/j.onehlt.2023.100669
- Piano Nazionale di Prevenzione Vaccinale 2017-2019. Accessed March 1, 2019. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Capelli G, Ravagnan S, Montarsi F, et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: a cost-effectiveness analysis in north-eastern Italy. Parasit Vectors. 2012;5(1):61. Published 2012 Mar 27. doi:10.1186/1756-3305-5-61