Tatjana Vilibić-Čavlek, Maja Bogdanić, Vladimir Savić, Ljubo Barbić, Vladimir Stevanović and Bernard Kaić
Key points
- The number of tick-borne diseases is increasing due to the geographical expansion of their tick vectors, higher frequencies of infected ticks, increased awareness of infection, and improved diagnostics.
- Ticks are vectors of numerous viruses (arboviruses), bacteria, and parasites.
- Tick-borne encephalitis (TBE) and Lyme disease (LD) are the most common and most widely distributed tick-borne infections in Europe. TBE is also endemic in northern and eastern Asia, while highly endemic areas for LD include the northeastern and north-central United States.
- The epidemiology of tick-borne infections differs according to the geographic region and season of the year.
- Clinical manifestations of tick-borne diseases vary from asymptomatic infection or mild febrile disease to hemorrhagic fever and neuroinvasive diseases.
- Diagnosis of tick-borne infections includes direct (cultivation, PCR/RT-PCR) and indirect methods (serology).
Introduction
Tick-borne diseases (TBDs) are emerging due to the geographical expansion of their tick vectors and represent an important public health problem worldwide.1 Ticks are vectors of a wide variety of viruses, bacteria, and parasites. Tick-borne viruses include a large group of arboviruses (mainly flaviviruses and bunyaviruses) with diverse genetic and pathogenic properties. Some arboviruses cause severe disease with a high case fatality rate in humans, while others may pose risks to public health, but their role in human diseases is still unclear or neglected.2 Clinical symptoms of tick-borne viral infections in humans range from mild fever to neuroinvasive diseases or hemorrhagic fevers.3 The medically most important tick-borne bacteria are Borrelia burgdorferi s.l. complex (Lyme disease; LD) and other Borrelia spp. (relapsing fever), spotted-fever Rickettsia spp., Anaplasma phagocytophilum (human granulocytic anaplasmosis; HGA), and Ehrlichia chaffeensis (human monocytic ehrlichiosis; HME). Babesiosis is the most common human tick-borne parasitic disease of increasing public health importance.1
Tick-borne flaviviruses are responsible for about 10,000 hospital admissions in Europe, Russia, China, and Japan each year. Between 10,000 and 15,000 cases of Crimean-Congo hemorrhagic fever (CCHF) are estimated to occur each year, mostly in bunyavirus endemic countries.1,4 LD is the most common tick-borne bacterial infection, with approximately 85,000 annual cases in Europe and 300,000 cases in the USA.1 According to epidemiological data, the number of HGA cases in the USA has increased significantly over time.5 Over three decades, there has been a noticeable increase in the identification of rickettsioses, mainly due to the advances in molecular diagnostics that have facilitated the identification of both previously recognized and novel rickettsia species.6 The number of Babesia microti infections has been on the rise in recent decades. More than 2000 cases of babesiosis are documented in the USA each year, however, the actual number is probably much higher.7 In addition, in the USA, babesiosis has been one of the main causes of transfusion-transmitted infections.8
This chapter focuses on the epidemiology and clinical characteristics of the most common medically important tick-borne viral, bacterial, and parasitic diseases.
Tick-borne viruses
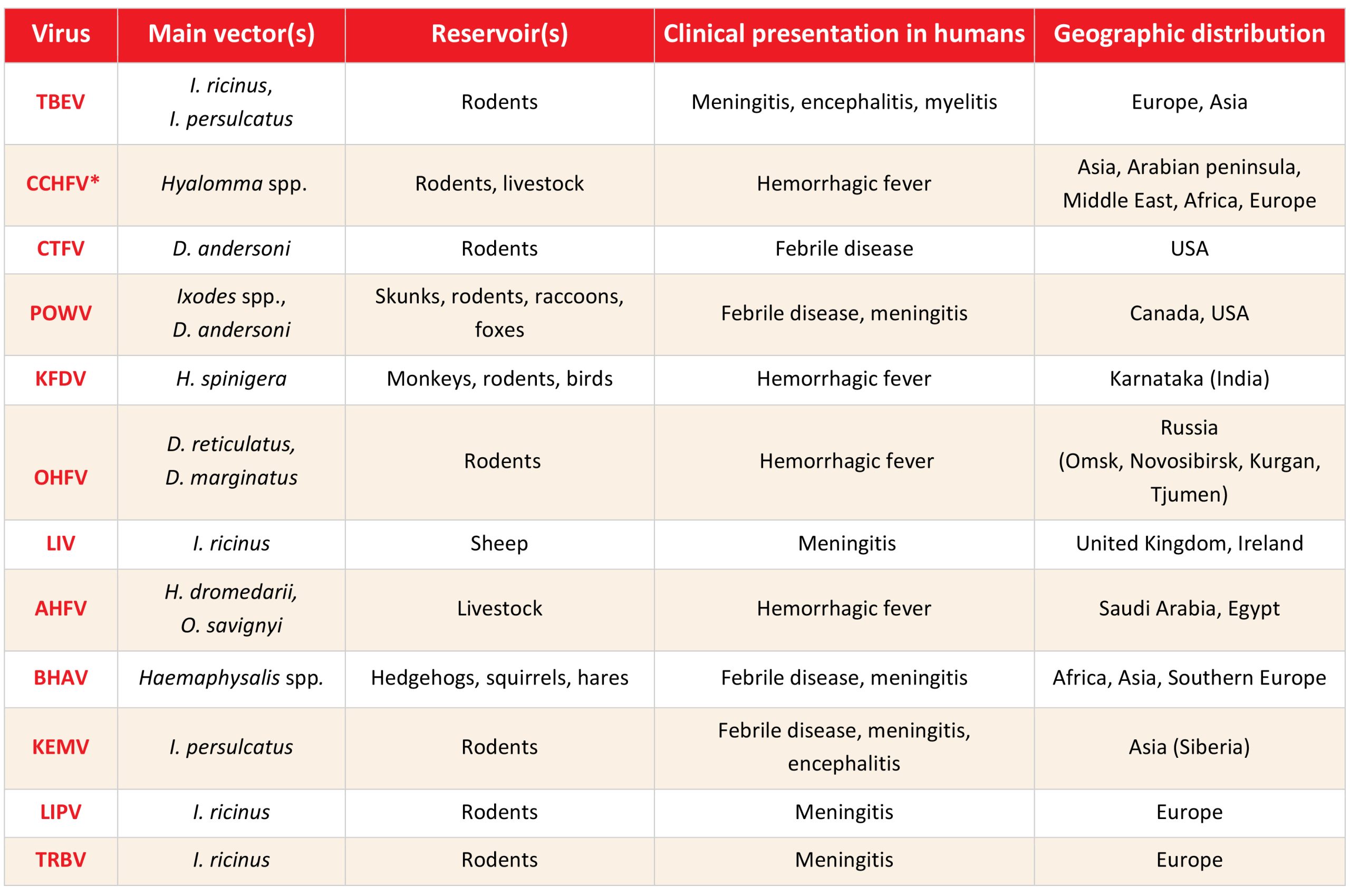
Among tick-borne arboviruses, tick-borne encephalitis virus (TBEV) is the most important human pathogen. Other medically important viruses include hemorrhagic fever viruses: Crimean-Congo hemorrhagic fever virus (CCHFV), Omsk hemorrhagic fever virus (OHFV), Kyasanur forest disease virus (KFDV) and Alkhumra hemorrhagic fever virus (AHFV) as well as other neurotropic arboviruses such as Powassan virus (POWV) and Louping ill virus (LIV). There are many other still neglected viruses such as Bhanja bandavirus (BHAV) and Kemerovo-related viruses. Severe fever with thrombocytopenia syndrome virus (SFTSV), Bourbon virus (BRBV), and Heartland virus (HRTV) are newly emerged tick-borne viruses (Table 1).1
Table 1: The most common tick-borne viruses of medical importance
Click the image above to enlarge
TBEV=tick-borne encephalitis virus, CCHFV=Crimean-Congo hemorrhagic fever virus, CTFV=Colorado tick fever virus, POWV=Powasan virus, KFDV=Kyasanur forest disease virus, OHFV=Omsk hemorrhagic fever virus, LIV=Louping ill virus; AHFV=Alkhumra hemorrhagic fever virus, BHAV=Bhanja bandavirus, KEMV=Kemerovo virus, LIPV=Lipovnik virus; TRBV=Tribec virus, *Interhuman transmission possible
Tick-borne encephalitis virus
TBEV (Orthoflavivirus encephalitidis virus, according to the latest ICTV classification) is the most widely distributed neurotropic arbovirus that belongs to the family Flaviviridae, genus Orthoflavivirus, tick-borne encephalitis serocomplex. Three main subtypes are European (TBEV-Eu), Far-East (TBEV-FE), and Siberian (TBEV-Sib). Ixodes ricinus is the main vector of the TBEV-Eu, while Ixodes persulcatus is a vector for TBEV-FE and TBEV-Sib.9,10 TBE is endemic in a large area from Central Europe and Scandinavia to Japan. Over the past two decades, the TBE incidence has increased in endemic areas; however, sporadic cases were also detected outside of known endemic regions. In many “non-endemic” areas of Eurasia, there are no commercial tests available or testing is not performed, therefore the possible cases are not reported. Human infections usually occur after a tick bite but the number of food-borne infections (consumption of unpasteurized goat milk) is increasing. The TBE-Eu is usually a biphasic disease. The first phase corresponds with viremia, while in the second phase symptoms of the central nervous system (CNS) occur (meningitis, encephalitis, myelitis). It is generally considered that TBEV-FE causes the most severe form of TBE and usually has a monophasic course. The case-fatality rate is 0.5-2% for the TBEV-Eu and 20% for the TBEV-FE.11 The TBE diagnosis is based on the detection of the intrathecal production of specific IgM antibodies or TBEV RNA.12
Crimean-Congo hemorrhagic fever virus
CCHFV is a bunyavirus of the family Nairoviridae, genus Orthonairovirus. CCHFV strains are classified into seven genotypes (I- VII). Ixodid ticks from the genus Hyalomma are the main vectors of CCHFV. Different wild and domestic animals, such as cattle, goats, sheep, and hares represent the virus reservoirs in nature.13 Humans become infected by a tick bite or exposure to body fluids from viremic animals or humans.2 People who have close contact with livestock (shepherds, farmers, butchers, slaughterhouse workers, and veterinarians) and those involved in outdoor activities (soldiers, farmers, forest workers, and hikers) are at high risk of exposure as well as healthcare personnel and close family members involved in patient care. CCHFV is widely distributed throughout Africa, the Middle East, Southeast Asia, and southern and eastern Europe. In humans, CCHF infections range from asymptomatic and mild infections (the majority of CCHFV cases) to severe and occasionally fatal hemorrhagic fever. In some regions, case fatality rates can be higher than 30%.14 RT-PCR and serology (IgM antibodies or a fourfold increase of IgG antibodies) are used for the diagnosis of CCHFV.4
Colorado tick fever virus
Colorado tick fever virus (CTFV) is a neglected virus that belongs to the family Spinareoviridae, genus Coltivirus. Transmission to humans occurs through a bite of the adult Rocky Mountain wood tick, Dermacentor andersoni. Both adults and nymphs are permanently infected, providing an overwintering mechanism for the virus.15 Because D. andersoni shows a broad host feeding preference, different vertebrate hosts have been identified as competent reservoirs for CTFV. The golden-mantled ground squirrel (Callospermophilus lateralis) is considered the most prominent natural reservoir of CTFV, while the other reservoirs include chipmunks, mice, rats, and hares. The CTFV is distributed in the western United States and southwestern Canada which correlates with the distribution of its tick vector. Human CTFV infections usually occur in the mid-summer when people are working or recreating in tick habitats. Infection in humans generally presents as a self-limiting febrile disease. Early diagnosis is primarily achieved using an RT-PCR or a 4-fold rise in IgG serology.16
Powassan virus
POWV is a tick-borne arbovirus of the family Flaviviridae, genus Orthoflavivirus. Two distinct genotypes are POWV lineage 1 and 2 (POWV-1 and POWV-1). Most human cases of POWV have been reported in the Great Lakes and Northeast regions of the USA and eastern Canada. In North America, the virus has been detected in four Ixodes species and Dermacentor andersoni ticks. The two enzootic cycles of POWV-1 include Ixodes cookei and groundhogs or mustelids, and Ixodes marxi and squirrels. POWV-2 is maintained in one enzootic cycle, primarily between Ixodes scapularis and the white-footed mouse.17 Unlike some other tick-borne pathogens, such as borrelia and babesia, which require tick attachment for 48 and 24 hours for transmission, POWV transmission can occur 15 to 50 minutes after ticks attach. In humans, POWV causes sporadic but severe encephalitis; however, the disease severity can vary significantly. Case fatality rates are ~20% in adults and ~7% in children. Long-term neurological complications are frequently observed in adults.18 The CSF serology is still the gold standard for confirmation of POWV neuroinvasive disease.19
Kyasanur forest disease virus
KFDV is a tick-borne arbovirus that belongs to the family Flaviviridae, genus Orthoflavivirus. After the first identification of KFDV in 1957 in monkeys from the Kyasanur Forest of Karnataka, India, 400-500 human cases have been reported annually. Haemaphysalis spinigera is the main vector of KFDV. Although the virus has been isolated from rodents, ground-dwelling birds, porcupines, cattle, and bats, only primates appear to develop the disease. Humans become infected by the bite of infected ticks or by handling of infecting mammals and birds.20 In humans, KFDV causes hemorrhagic fever with a case fatality rate of 3-5%. Some patients (10-20%) develop a secondary phase of fever relapse with meningoencephalitis. Diagnosis is usually confirmed by RT-PCR in a blood sample. Humans usually show high-level viremia (about 106 pfu/mL) around day 3 after the onset of symptoms that persist for up to two weeks. The ELISA can be used for the detection of IgM and IgG antibodies.21 A formalin-inactivated whole KFDV vaccine produced in chick embryo fibroblasts is available.22
Omsk hemorrhagic fever virus
OHFV is an arbovirus closely related to TBEV (family Flaviviridae, genus Orthoflavivirus). Humans become infected through tick bites or contact with the blood, feces, or urine of infected rodents, mainly muskrats (Ondatra zibethicus).23 The disease is prevalent in four regions of western Siberia in Russia (Kurgan, Tyumen, Omsk, and Novosibirsk). The Ixodidae ticks Dermacentor reticulatus and Dermacentor marginatus are the main hosts for OHFV in the forests and steppes of Siberia. Very recently, the OHFV RNA has been detected in the CSF of two patients from Almaty, Kazakhstan. In addition, the virus was detected in ticks in the Akmola region in Kazakhstan. The disease occurs mainly in muskrat trappers (60%). Hunters are at risk of infection when skinning infected animals. Omsk hemorrhagic fever (OHF) is a self-limiting acute disease in most cases, although a small proportion progresses to hemorrhagic disease. The fatality of OHF is low (0.5-3%). Diagnosis of OHF is based on RT-PCR, OHFV-NS1 antigen detection, and serology.24 Data suggest that the TBE vaccination provides a high degree of protection against OHF.25
Louping ill virus
Louping ill virus (LIV) is a tick-borne arbovirus closely related to TBEV, and belongs to the Flaviviridae family, genus Orthoflavivirus. Although LIV has previously been found exclusively on the British Islands, it has recently been discovered in Norway and on the Danish island of Bornholm in the Baltic Sea. Ixodes ricinus is the only known tick vector for LIV while sheep, mountain hares, and red grouse are the most important hosts.26 Human infections caused by LIV are rare and occur after a tick bite or occupational exposure to infected sheep tissues. Risk groups include professionally exposed individuals who have contact with sheep or other potentially infected animals, such as abattoir workers, butchers, and veterinarians. LIV infections in humans are mostly asymptomatic or present as a flu-like disease, while mild meningoencephalitis is rare.27
Alkhumra hemorrhagic fever virus
AHFV is a tick-borne virus of the family Flaviviridae, genus Orthoflavivirus. The virus was first isolated in 1995 from a 32‑year‑old male butcher from Alkhumra district (Jeddah, Saudi Arabia), who died of hemorrhagic fever. Since then, AHFV cases have been reported among residents of Saudi Arabia and tourists in Egypt and Djibouti. The AHFV epidemiology is not fully understood. Epidemiological studies have shown that AHFV cases were linked to direct or indirect contact with infected blood/organs of slaughtered livestock and ingestion of infected raw milk. The transmission through a tick bite has also been reported in the literature. The hard tick Hyalomma dromedarii and the soft tick Ornithodoros savignyi are potential vectors of AHFV.28 Clinical symptoms in humans range from subclinical or mild to severe and rapidly fatal infection.29 Acute febrile flu-like illness, hepatitis, and hemorrhagic manifestations are the main clinical features of AHFV infection. Mortality in hospitalized patients may reach 30%. RT-PCR or serology can confirm the diagnosis.28
Kemerovo related viruses
The Kemerovo serogroup (family Reoviridae, genus Orbivirus) contains more than 50 tick-borne viruses of which only Kemerovo virus (KEMV), Lipovnik virus (LIPV), and Tribeč virus (TRBV) have been associated with human diseases. An illness caused by the KEMV virus was first described in the taiga landscape in the Kemerovo region in Western Siberia in 1962, where the virus was isolated from ticks and the CSF of patients with meningitis and meningoencephalitis after a tick bite. In a natural cycle, rodents are reservoirs and I. persulcatus tick is a vector of KEMV. In humans, KEMV causes febrile disease and occasionally meningitis.30,31 LIPV was isolated from I. ricinus ticks collected in 1963 in Lipovnik village, Slovakia. Meningoencephalitis and polyradiculitis have been linked to LIPV in the Czech Republic. TRBV was isolated in 1963 from I. ricinus ticks and the blood of small rodents in the Tribeč mountains, Slovakia.32 A TRBV was detected from Siberia to central Europe by virus isolation from ticks and antibodies detected in animals. In humans, TRBV-specific antibodies were detected in patients with febrile disease and meningitis.30,33,34
Bhanja bandavirus
BHAV is a neglected tick-borne bunyavirus of the family Phenuiviridae, genus Bandavirus. The virus was isolated in 1954 from the Haemaphysalis intermedia tick collected from goats in Bhanjanagar, India, while the first human case of BHAV infection was reported in 1974. BHAV is widely distributed in central Europe, the Mediterranean basin, the Middle East to India, and in Sub-Saharan Africa, however, human clinical infections are rare. The natural reservoirs of BHAV are sheep, goats, hares, hedgehogs, and squirrels, while Haemaphysalis ticks are the main vectors in Europe.11 Only a few human cases of neuroinvasive diseases caused by BHAV have been reported.35,36 RT-PCR and serology are used for the diagnosis of BHAV infection.11
Dabie bandavirus (Severe fever with thrombocytopenia syndrome virus)
SFTSV is one of the emerging pathogenic tick-borne viruses reported in patients with severe fever, thrombocytopenia, and leukocytopenia and an initial fatality rate of up to 30%.37 SFTSV was first discovered in China (2009) and later in South Korea and Japan. Some patients reported a history of tick bites, and the virus was detected primarily in Haemaphysalis longicornis ticks originating from regions where the patients lived.38 Several studies indicated that infected patients can spread the virus to family members or healthcare workers, primarily through contact with contaminated blood or body fluids.39 Hemorrhagic fever with thrombocytopenia, leukocytopenia, and increased liver enzymes are the main clinical and laboratory findings in patients with severe SFTSV infection. Fatalities mainly occur in patients over 50, with mortality rates ranging from 10 to 19%. RT-PCR is the gold standard diagnostic method for the detection of SFTSV.40
Bourbon virus
Bourbon virus (BRBV) is a recently discovered tick-borne virus of the genus Togotovirus, family Orthomyxoviridae that was first identified in a fatal human case in Bourbon County, Kansas, USA in 2014. The virus has been associated with several cases of severe acute febrile illness in patients in the Midwest US, but since 2020, the BRBV has been reported in North Carolina, Virginia, New Jersey, and New York State. Amblyomma americanum is considered to be the primary vector of BRBV, while the mammalian reservoir has not been identified yet. However, serological testing has identified white-tailed deer and raccoons as potential sentinels to track the spread of BRBV. Clinical symptoms of BRBV infection include fever, weakness, fatigue, myalgia, arthralgia, and nausea that occur 2-7 days after a tick bite. Shock, organ failure, cardiac dysregulation, pleural effusions, and acute bone marrow suppression were linked to fatal cases. RT-PCR is used to diagnose the BRBV.41-43
Heartland virus
Heartland virus (HRTV) is an emerging bunyavirus first discovered in the USA in 2009. Originally classified in the genus Phlebovirus, family Phenuiviridae, the virus is now reclassified in the Bandavirus genus alongside BHAV and SFTSV. HRTV infections are reported mainly east of the Mississippi River, mostly in the summer months. The Lone Star tick, Amblyomma americanum is considered the primary vector of HRTV zoonotic transmission. It is also possible that Amblyomma or Haemaphysalis tick species are the sole reservoirs of HRTV. Numerous possible amplification hosts, including raccoons, white-tailed deer, coyotes, domestic dogs, and opossums, have been identified based on serosurveillance studies. However, clinical infections have been reported only in humans.44 Clinical symptoms of HRTV infection include fever, headache, fatigue, myalgia, nausea, and diarrhea with leucopenia and thrombocytopenia. RT-PCR is most commonly used for the diagnosis of HRTV. The plaque reduction neutralization test (PRNT) is used for screening both human and animal serum samples in serosurveillance studies.45
Tick-borne bacteria
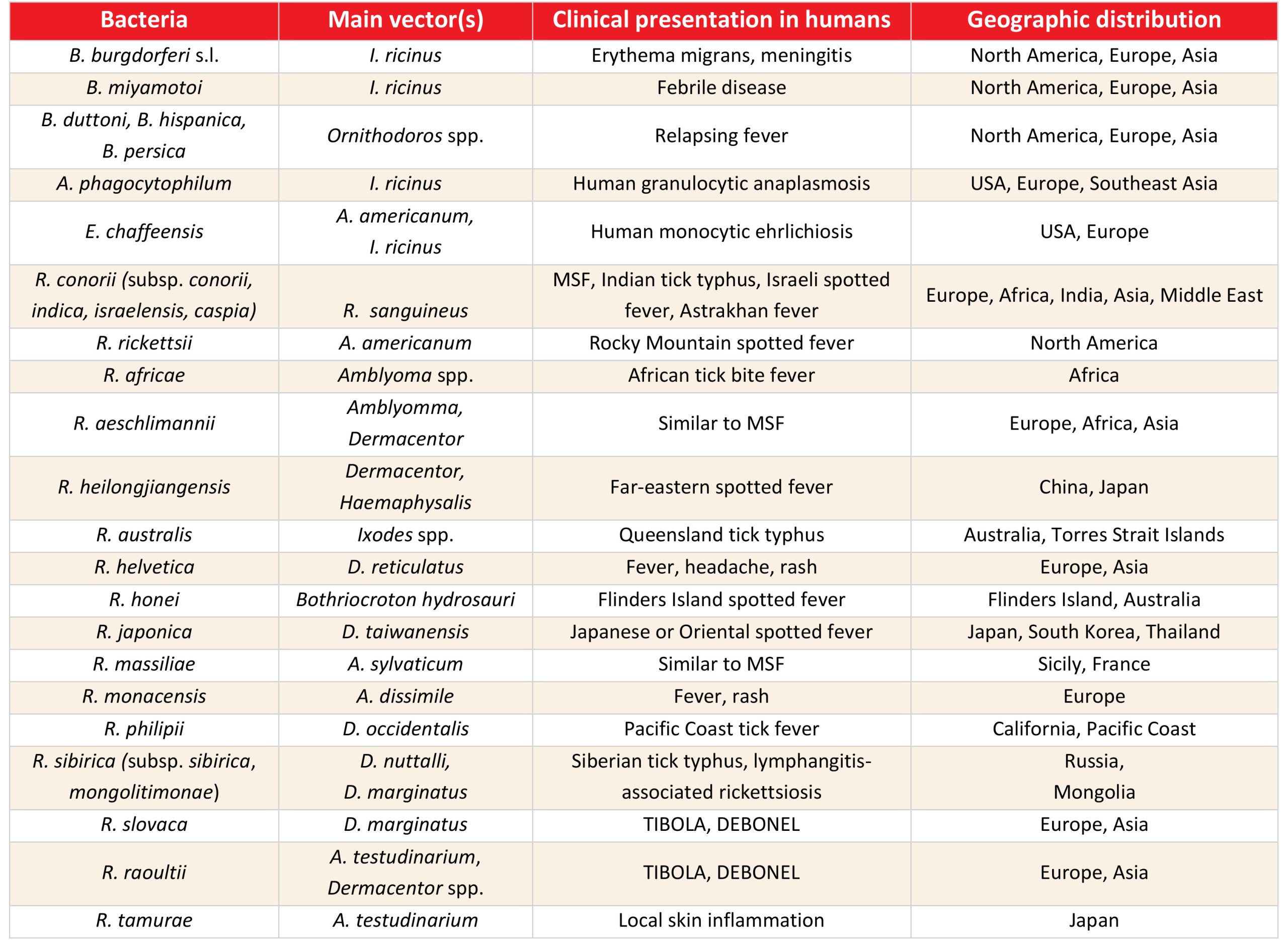
Borrelia burgdorferi s.l., a causative agent of LB is the most frequently detected tick-borne bacteria with a worldwide distribution.46 Cases of HGA have been identified in the upper Midwest and the Northeast USA, Northern Europe, and Southeast Asia.47 The majority of HME cases in the USA are caused by E. chaffeensis.48 Spotted-fever group (SFG) rickettsia are a neglected group of bacteria of the genus Rickettsia, family Rickettsiaceae that includes numerous emerging infectious diseases with a worldwide distribution.49 The main tick-borne bacteria are presented in Table 2.
Borrelia spp.
The three main species of Borrelia burgdorferi sensu lato (s.l.) complex associated with human LD are B. burgdorferi sensu stricto (s.s.), Borrelia afzelii and Borrelia garinii. Ixodes ricinus is the main tick vector in Europe. Ixodes persulcatus and Ixodes hexagonus are also proven vectors of B. burgdorferi s.l. Rodents are the principal reservoir hosts of borrelia. Clinical manifestations of LD may be localized (erythema migrans) or disseminated (arthritis, carditis, neuroborreliosis).50 Serology tests (ELISA, IFA, immunoblot) for the detection of borrelia antibodies in the blood or CSF are most commonly used for the diagnosis of LD. Therapy of LD depends on the patient’s age and the stage of the disease. Doxycycline is recommended for patients older than 8 years with localized disease. Patients under the age of 8 should receive amoxicillin or cefuroxime. Parenteral therapy may be required for more severe manifestations such as arthritis, carditis, meningitis, or encephalitis.51
Relapsing fever (RF) is another tick-borne borreliosis distributed in the Northern Hemisphere, Africa, and Central America. Borrelia duttoni, B. hispanica, and B. persica are the main tick-borne borreliae transmitted by soft-bodied or argasid ticks. Small rodents and other mammals, including bats serve as a reservoir for tick-borne Borrelia species.52 Clinical symptoms of RF typically include a high fever for a few days followed by a period of well-being and another relapse. Without antibiotic therapy, relapses can occur several times.53 The diagnosis of RF can be confirmed by direct microscopic detection of borrelia in Giemsa-stained blood films, serologic analysis, or PCR. RF is treated with doxycycline. Penicillin or erythromycin are preferred in pregnant women and children under 8 years of age.52
Borrelia miyamotoi is a new tick-borne Borrelia species discovered in Japan in 1995. The pathogenicity was suggested in 2011 in Russia when 51 patients with suspected tick bites developed a nonspecific febrile illness and B. miyamotoi was confirmed by PCR or specific antibodies. Immunocompetent individuals present with a mild flu-like disease, but the disease may be more severe in immunocompromised patients. PCR that detects B. miyamotoi DNA in blood or CSF and serologic assays are used for disease confirmation.54 Borrelia miyamotoi infections are treated with doxycycline. Amoxicillin and ceftriaxone have also been successfully used for the treatment of B. miyamotoi.55
Table 2: Epidemiological and clinical characteristics of the most common tick-borne bacteria
Click the image above to enlarge
TIBOLA= tick-borne lymphadenitis, DEBONEL= dermacentor-borne necrosis erythema lymphadenopathy
Anaplasma phagocytophilum
A. phagocytophilum, an obligate intracellular bacteria is the most important species within the Anaplasma genus that causes HGA. Ixodes ricinus tick is the main vector of HGA in Europe, while I. scapularis and I. pacificus are vectors in the USA.56 Whereas some patients with HGA remain asymptomatic, others develop a nonspecific febrile disease, and only a small proportion develop severe disease. The most common symptoms of HGA include fever, headache, malaise, myalgia, and arthralgia. The mortality rate is about 0.6%. Whole-blood PCR is the most sensitive method to diagnose HGA. A Giemsa-stained peripheral blood smear may reveal morulae within the polymorphonuclear leukocytes. IFA can be used for the detection of specific IgM and/or IgG antibodies.5 Doxycycline is the recommended first-line therapy for HGA.47
Ehrlichia spp.
The genus Ehrlichia includes several tick-borne obligate intracellular bacteria that infect humans and other mammals. The most important species are Ehrlichia chaffeesis, which causes HME, and Ehrlichia ewingii, which causes Ehrlichia ewingii ehrlichiosis. The Lone Star tick (A. americanum) is the most common vector in the USA,48 while I. ricinus is a vector in Europe.57 Ehrlichia infections are reported most often in the elderly. Since children frequently develop milder or subclinical infections, the disease is probably underreported in this population group. Patients with ehrlichiosis typically present with a flu-like febrile disease. CNS involvement including meningitis and meningoencephalitis occurs in up to 20% of patients.48 The overall case fatality rate is 1%. Diagnosis of ehrlichiosis is usually confirmed using PCR or serology. Tetracyclines are highly efficacious for the therapy of ehrlichiosis.58
Rickettsia spp.
Tick-borne rickettsioses are caused by obligate intracellular bacteria belonging to the spotted fever group (SFG) of the Rickettsia genus. The most widely distributed SFG rickettsia include Rickettsia rickettsii (Rocky Mountain spotted fever; RMSF), R. conorii (Mediterranean spotted fever; MSF), R. africae (African tick bite fever), R. helvetica, R. aeschlimannii, R. slovaca (tick-borne lymphadenitis; TIBOLA Dermacentor-borne necrosis erythema lymphadenopathy; DEBONEL), and R. raoultii.6,59 In addition to pathogenic rickettsia species, there are many potentially pathogenic “candidates” for new species. Most SFG rickettsiae are transmitted by ixodid tick bites during blood feeding. The distribution of SFG rickettsioses varies geographically and correlates with the distribution of tick vectors.6 Localized rickettsial infections appear as an eschar (also known as a “tache noir“) at the site of tick inoculation. However, disseminated infection can cause severe vasculitis and endothelial damage, which can manifest as cutaneous necrosis and digital gangrene, pneumonitis, meningoencephalitis, and multiorgan failure.60 Serology (IFA) is the most commonly used for the diagnosis of rickettsioses. PCR enables species-specific identification.61 Doxycycline is the therapy of choice for SFG rickettsial diseases.62
Tick-borne parasites
Babesia microti, B. divergens, B. duncani and B. venatorum are the main zoonotic babesia species that can cause human diseases. Babesia microti is the most reported species in North America, while B. divergens is the most common cause of human babesiosis in Europe. The tick vectors of babesia include I. scapularis (North America), I. ricinus (Europe), and I. persulcatus (Asia). Babesiosis is typically asymptomatic and self-limiting in healthy individuals. However, in elderly, splenectomised, and other immunocompromised individuals the disease may be severe with hemolytic anemia, splenomegaly, hepatomegaly, and renal failure, sometimes with fatal outcomes.63 Peripheral thick and thin blood smear examination has been the standard method for diagnosing human babesiosis. Serological tests (EIA, IFA, IB) have been used to support or confirm the diagnosis of babesiosis in endemic regions. PCR targeting the Babesia spp. is 18S rRNA can also be used.64 The current therapy for human babesiosis includes combinations of atovaquone and azithromycin or clindamycin and quinine.65
Concluding remarks
The number of TBDs is increasing, and this trend is expected to continue. Based on information from animal experiments, a large number of potential tick-borne pathogens have already been proposed. It was also noted that the clinical spectrum of TBDs is becoming more diverse, including underrecognized manifestations of previous well-known pathogens. To effectively develop strategies to mitigate the increasing incidence of TBDs, a deeper understanding of the ecological and biological factors driving the expansion of tick vectors and reservoir host distributions, as well as the microbiological dynamics within ticks that modulate pathogen emergence, is required.66
Contact
Tatjana Vilibić-Čavlek
tatjana.vilibic-cavlek@hzjz.hr
Authors
Tatjana Vilibić-Čavlek, Maja Bogdanić, Vladimir Savić, Ljubo Barbić, Vladimir Stevanović and Bernard Kaić
Citation
Vilibić-Čavlek T, Bogdanić M, Savić V, Barbić L, Stevanović V, Kaić B. Tick-borne human diseases around the globe. Chapter 1. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ, eds. The TBE Book. 7th ed. Singapore: Global Health Press; 2024. doi:10.33442/26613980_1-7
References
- Rochlin I, Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J Med Microbiol. 2020;69(6):781-791. doi:10.1099/jmm.0.001206.
- Shi J, Hu Z, Deng F, Shen S. Tick-Borne Viruses. Virol Sin. 2018;33:21-43. doi:10.1007/s12250-018-0019-0.
- Shah T, Li Q, Wang B, Baloch Z, Xia X. Geographical distribution and pathogenesis of ticks and tick-borne viral diseases. Front Microbiol. 2023;14:1185829. doi:10.3389/fmicb.2023.1185829.
- Raabe VN. Diagnostic Testing for Crimean-Congo Hemorrhagic Fever. J Clin Microbiol. 2020;58(4):e01580-19. doi:10.1128/JCM.01580-19.
- Dumic I, Jevtic D, Veselinovic M, et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms. 2022;10(7):1433. doi:10.3390/microorganisms10071433.
- Piotrowski M, Rymaszewska A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms. 2020;8(12):1906. doi:10.3390/microorganisms8121906.
- Kumar A, O’Bryan J, Krause PJ. The Global Emergence of Human Babesiosis. Pathogens. 2021;10(11):1447. doi:10.3390/pathogens10111447.
- Bloch EM, Krause PJ, Tonnetti L. Preventing Transfusion-Transmitted Babesiosis. Pathogens. 2021;10:1176. doi:10.3390/pathogens10091176.
- Kwasnik M, Rola J, Rozek W. Tick-Borne Encephalitis-Review of the Current Status. J Clin Med. 2023;12(20):6603. doi:10.3390/jcm12206603.
- Pustijanac E, Buršić M, Talapko J, Škrlec I, Meštrović T, Lišnjić D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms. 2023;11(7):1634. doi:10.3390/microorganisms11071634.
- Vilibić-Čavlek T, Savić V, Židovec-Lepej S, Bogdanić M, Stevanović V, Barbić Lj. Emerging and neglected viral zoonoses in Europe. In: Rodriguez-Morales AJ, ed. Current Topics in Zoonoses. IntechOpen: London, United Kingdom, 2023; doi:10.5772/intechopen.112779
- Phipps LP, Johnson N. Tick-borne encephalitis virus. J Med Microbiol. 2022;71(5). doi:10.1099/jmm.0.001492.
- Centers for Disease Control and Prevention (CDC). Crimean-Congo Hemorrhagic Fever. Available from: https://www.cdc.gov/vhf/crimean-congo/transmission/index.html
- Hawman DW, Feldmann H. Crimean-Congo haemorrhagic fever virus. Nat Rev Microbiol. 2023;21(7):463-477. doi:10.1038/s41579-023-00871-9.
- Centers for Disease Control and Prevention (CDC). Colorado tick fever. Available from: https://www.cdc.gov/coloradotickfever/index.html
- Harris EK, Foy BD, Ebel GD. Colorado tick fever virus: a review of historical literature and research emphasis for a modern era. J Med Entomol. 2023;60(6):1214-1220. doi:10.1093/jme/tjad094.
- Corrin T, Greig J, Harding S, Young I, Mascarenhas M, Waddell LA. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health. 2018;65(6):595-624. doi:10.1111/zph.12485.
- Kakoullis L, Vaz VR, Kaur D, et al. Powassan Virus Infections: A Systematic Review of Published Cases. Trop Med Infect Dis. 2023; 8(12):508. doi:10.3390/tropicalmed8120508.
- Kapoor AK, Zash R. Powassan Virus. [Updated 2023 Mar 27] [Accessed 2024 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570599/
- Bhatia B, Feldmann H, Marzi A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus-Two Neglected Zoonotic Pathogens. Microorganisms. 2020;8(9):1406. doi:10.3390/microorganisms8091406.
- Chunduru K, Saravu K. Kyasanur forest disease: A review on the emerging infectious disease. J Clin Infect Dis Soc. 2023;1:5-11.
- Srikanth UGK, Marinaik CB, Gomes AR, et al. Evaluation of Safety and Potency of Kyasanur Forest Disease (KFD) Vaccine Inactivated with Different Concentrations of Formalin and Comparative Evaluation of In Vitro and In Vivo Methods of Virus Titration in KFD Vaccine. Biomedicines. 2023;11(7):1871. doi:10.3390/biomedicines11071871.
- Kovalev SY, Mazurina EA. Omsk hemorrhagic fever virus is a tick-borne encephalitis virus adapted to muskrat through host-jumping. J Med Virol. 2022;94(6):2510-2518. doi:10.1002/jmv.27581.
- Wagner E, Shin A, Tukhanova N, et al. First Indications of Omsk Haemorrhagic Fever Virus beyond Russia. Viruses. 2022;14(4):754. doi:10.3390/v14040754.
- Chidumayo NN, Yoshii K, Kariwa H. Evaluation of the European tick-borne encephalitis vaccine against Omsk hemorrhagic fever virus. Microbiol Immunol. 2014;58(2):112-118. doi:10.1111/1348-0421.12122.
- Ytrehus B, Rocchi M, Brandsegg H, et al. Louping-ill virus serosurvey of willow ptarmigan (Lagopus lagopus lagopus) in Norway. J Wildl Dis. 2021;57(2):282-291. doi:10.7589/JWD-D-20-00068.
- Jeffries CL, Mansfield KL, Phipps LP, Wakeley PR, Mearns R, Schock A, Bell S, Breed AC, Fooks AR, Johnson N. Louping ill virus: an endemic tick-borne disease of Great Britain. J Gen Virol. 2014;95(Pt 5):1005-1014. doi:10.1099/vir.0.062356-0.
- Abdulhaq AA, Hershan AA, Karunamoorthi K, Al-Mekhlafi HM. Human Alkhumra hemorrhagic Fever: Emergence, history and epidemiological and clinical profiles. Saudi J Biol Sci. 2022;29(3):1900-1910. doi:10.1016/j.sjbs.2021.10.031.
- Madani TA, Abuelzein EME. Alkhumra hemorrhagic fever virus infection. Arch Virol. 2021;166(9):2357-2367. doi:10.1007/s00705-021-05083-1.
- Peňazziová K, Korytár Ľ, Cingeľová Maruščáková I, et al. Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds. Microorganisms. 2022;10(12):2397. doi:10.3390/microorganisms10122397.
- Migné CV, Braga de Seixas H, Heckmann A, et al. Evaluation of Vector Competence of Ixodes Ticks for Kemerovo Virus. Viruses. 2022;14(5):1102. doi:10.3390/v14051102.
- Belhouchet M, Mohd Jaafar F, Tesh R, et al. Complete sequence of Great Island virus and comparison with the T2 and outer-capsid proteins of Kemerovo, Lipovnik and Tribec viruses (genus Orbivirus, family Reoviridae). J Gen Virol. 2010;91(Pt 12):2985-2993. doi:10.1099/vir.0.024760-0.
- Dilcher M, Hasib L, Lechner M, et al. Genetic characterization of Tribeč virus and Kemerovo virus, two tick-transmitted human-pathogenic Orbiviruses. Virology. 2012; 423(1):68-76. doi:10.1016/j.virol.2011.11.020.
- Hubálek Z. History of Arbovirus Research in the Czech Republic. Viruses. 2021; 13(11):2334. doi:10.3390/v13112334.
- Calisher CH, Goodpasture HC. Human infection with Bhanja virus. Am J Trop Med Hyg. 1975;24(6 Pt 1):1040-1042. doi:10.4269/ajtmh.1975.24.1040.
- Vilibic-Cavlek T, Stevanovic V, Krcmar S, et al. Detection of Bhanja Bandavirus in Patients with Neuroinvasive Disease of Unknown Etiology in Croatia. Microorganisms. 2023;11(9):2155. doi:10.3390/microorganisms11092155.
- Li J, Li S, Yang L, Cao P, Lu J. Severe fever with thrombocytopenia syndrome virus: a highly lethal bunyavirus. Crit Rev Microbiol. 2021;47(1):112-125. doi:10.1080/1040841X.2020.1847037.
- Luo LM, Zhao L, Wen HL, et al. Haemaphysalis longicornis Ticks as Reservoir and Vector of Severe Fever with Thrombocytopenia Syndrome Virus in China. Emerg Infect Dis. 2015;21(10):1770-1776. doi:10.3201/eid2110.150126.
- Bae S, Chang HH, Kim SW, et al. Nosocomial outbreak of severe fever with thrombocytopenia syndrome among healthcare workers in a single hospital in Daegu, Korea. Int J Infect Dis. 2022;119:95-101. doi:10.1016/j.ijid.2022.03.048.
- Kim EH, Park SJ. Emerging Tick-Borne Dabie Bandavirus: Virology, Epidemiology, and Prevention. Microorganisms. 2023;11(9):2309. doi:10.3390/microorganisms11092309.
- Lange RE, Dupuis AP 2nd, Ciota AT. Diversification of Bourbon Virus in New York State. Microorganisms. 2023; 11(6):1590. doi:10.3390/microorganisms11061590.
- Roe MK, Huffman ER, Batista YS, et al. Comprehensive Review of Emergence and Virology of Tickborne Bourbon Virus in the United States. Emerg Infect Dis. 2023;29(1):1-7. doi:10.3201/eid2901.212295.
- Bendl E, Fuchs J, Kochs G. Bourbon virus, a newly discovered zoonotic thogotovirus. J Gen Virol. 2023;104(8). doi:10.1099/jgv.0.001887.
- Mantlo EK, Haley NJ. Heartland Virus: An Evolving Story of an Emerging Zoonotic and Vector-Borne Disease. Zoonotic Dis. 2023;3:188-202. doi:10.3390/zoonoticdis3030016
- Dembek ZF, Mothershead JL, Cirimotich CM, Wu A. Heartland Virus Disease—An Underreported Emerging Infection. Microorganisms. 2024;12(2):286. doi:10.3390/microorganisms12020286.
- Marques AR, Strle F, Wormser GP. Comparison of Lyme disease in the United States and Europe. Emerg Infect Dis. 2021; 27(8):2017-2023. doi:10.3201/eid2708.204763.
- Guzman N, Yarrarapu SNS, Beidas SO. Anaplasma phagocytophilum. [Updated 2023 Aug 8] [Accessed 2024 Mar 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513341/
- Snowden J, Bartman M, Kong EL, et al. Ehrlichiosis. [Updated 2022 Sep 12] [Accessed 2024 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441966/
- Robinson MT, Satjanadumrong J, Hughes T, Stenos J, Blacksell SD. Diagnosis of spotted fever group Rickettsia infections: the Asian perspective. Epidemiol Infect. 2019; 147:e286. doi:10.1017/S0950268819001390.
- Steinbrink A, Brugger K, Margos G, Kraiczy P, Klimpel S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol Res. 2022; 121(3):781-803. doi:10.1007/s00436-022-07445-3.
- Skar GL, Simonsen KA. Lyme Disease. [Updated 2024 Feb 4] [Accessed 2024 Jan 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Snowden J, Yarrarapu SNS, Oliver TI. Relapsing Fever. [Updated 2023 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441913/
- Centers for Disease Control and Prevention (CDC). Tick- and Louse-Borne Relapsing Fever. Available from: https://www.cdc.gov/relapsing-fever/symptoms/index.html
- Cleveland DW, Anderson CC, Brissette CA. Borrelia miyamotoi: A Comprehensive Review. Pathogens. 2023;12(2):267. doi:10.3390/pathogens12020267.
- Centers for Disease Control and Prevention (CDC). Borrelia miyamotoi. Available from: https://www.cdc.gov/relapsing-fever/miyamotoi/index.html
- Matei IA, Estrada-Peña A, Cutler SJ, et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. 2019;12(1):599. doi:10.1186/s13071-019-3852-6.
- Stanilov I, Blazhev A, Miteva L. Anaplasma and Ehrlichia Species in Ixodidae Ticks Collected from Two Regions of Bulgaria. Microorganisms. 2023;11(3):594. doi:10.3390/microorganisms11030594.
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45 Suppl 1:S45-51. doi:10.1086/518146.
- Parola P, Paddock CD, Socolovschi C, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26(4):657-702. doi:10.1128/CMR.00032-13.
- Abdad MY, Abou Abdallah R, Fournier P-E, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. 2018;56:e01728-17. doi:10.1128/JCM.01728-17.
- Stewart AG, Stewart AGA. An Update on the Laboratory Diagnosis of Rickettsia spp. Infection. Pathogens. 2021;10(10):1319. doi:10.3390/pathogens10101319.
- Binder AM, Armstrong PA. Patient characteristics, treatment patterns, and outcomes of Rickettsial diseases among a commercially insured population in the United States, 2005-2017. Sci Rep. 2021;11(1):18382. doi:10.1038/s41598-021-96463-9.
- Young KM, Corrin T, Wilhelm B, Uhland C, Greig J, Mascarenhas M, Waddell LA. Zoonotic Babesia: A scoping review of the global evidence. PLoS One. 2019; 14(12):e0226781. doi:10.1371/journal.pone.0226781.
- Parija SC, Kp D, Venugopal H. Diagnosis and management of human babesiosis. Trop Parasitol. 2015;5(2):88-93. doi:10.4103/2229-5070.162489.
- Renard I, Ben Mamoun C. Treatment of Human Babesiosis: Then and Now. Pathogens. 2021;10(9):1120. doi:10.3390/pathogens10091120.
- Paddock CD, Lane RS, Staples JE, et al. Changing paradigms for tick-borne diseases in the Americas. In: Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. Washington (DC): National Academies Press (US); 2016 Sep 21. A8. Available from: https://www.ncbi.nlm.nih.gov/books/NBK390439/