Wilhelm Erber, Michael Broeker, Gerhard Dobler, Lidia Chitimia-Dobler, Heinz-Josef Schmitt
Key points
- TBE is a flavivirus infection of the central nervous system (CNS), transmitted by ticks and in some rare instances by ingestion of unpasteurized milk
- It is diagnosed in the Boreal and Temperate Forest Belt of Eurasia ranging from the UK, eastern France, The Netherlands and Norway down to Italy through central and Eastern Europe, Russia, Kazakhstan, and China to Japan.
- About 10,000 cases of TBE are reported annually, likely a significant underestimate as serological testing is more sporadic than complete and, in some countries, (like Japan) not even available.
- The European Centre for Disease Prevention and Control (ECDC) have put TBE on their list of notifiable diseases. Their case definition requires clinical symptoms of CNS infection plus virological or serological confirmation of the infection, usually by detection of specific immunoglobulins IgG and IgM.
- Vaccination against TBE is on the World Health Organization’s List of Essential Medicines, the safest and most effective medicines needed in a health system.
- Surveillance of TBE and the TBEV is incomplete. Reported incidences do not reflect actual risk since this fluctuates annually as a result of changes in exposure, vaccine uptake, intensity of case finding and reporting, climate factors, reservoir animals and ticks – just to mention the most relevant factors.
- For largely unknown reasons (including human behavior, improved diagnostics, or climate change) TBEV appears to be spreading north (e.g. northern Scandinavia), west (e.g. United Kingdom, even south (e.g. Tunisia) and to higher altitudes (e.g. in the Alps) to areas that were previously believed to be free of the virus.
- The vectors for TBE virus are ticks like Ixodes ricinus and Ixodes persulcatus. Reservoir animals for TBE virus are mainly small rodents.
1. Burden of disease and case definition
Since the first description of the clinical symptoms of TBE and the detection of TBEV in Far Eastern Russia nearly a century ago,1 TBE has become the most important tick-borne viral disease across Eurasia. To date, tick-borne encephalitis virus (TBEV) foci have been identified in Europe, Russia, through to northern and eastern Asia up to Japan. Up to 12,000 human tick-borne encephalitis (TBE) cases are registered annually from countries where the disease is reportable. However, this number likely represents an underestimate due to under-diagnosis and/or underreporting. Case fatality rates between 0.2% to 20% are reported, depending on region and perhaps on viral subtype.2 Severe long-term sequelae of TBE are well described both in children and in adults (see Chapters 8 and 9).
1.1. TBE case definition
Because TBEV is present in reservoir animals in nature, eliminating or eradicating the disease is impossible. Thus TBE is an important concern for the potentially exposed individual who becomes infected, but it is also of public health relevance, as acknowledged by the World Health Organization (WHO) in all position reports from 1983 to date (2011).3-5 Moreover, TBE vaccination against TBE is on the World Health Organization’s List of Essential Medicines, the safest and most effective medicines needed in a healthcare system.6 In addition, in 2012 the European Center for Disease Prevention and Control (ECDC) decided to add TBE to the list of mandatory notifiable diseases and provided for the first time ever a uniform disease case definition (Table 1).7
Table 1: TBE case definition by the ECDC4
- Clinical Criteria
Any person with symptoms of inflammation of the CNS (e.g. meningitis, meningoencephalitis, encephalomyelitis, encephaloradiculitis)
- Laboratory Criteria
Laboratory criteria for case confirmation:*
At least one of the following five:
– TBE specific IgM AND IgG antibodies in blood
– TBE specific IgM antibodies in CSF
– Sero-conversion or four-fold increase of TBE-specific antibodies in paired serum samples
– Detection of TBE viral nucleic acid in a clinical specimen,
– Isolation of TBE virus from clinical specimen
Laboratory criteria for a probable case:
Detection of TBE-specific IgM-antibodies in a unique serum sample
- Epidemiological Criteria
Case Classification
1. Possible case (Not applicable)
2. Probable case
Any person meeting the clinical criteria and the laboratory criteria for a probable case
OR Any person meeting the clinical criteria and with an epidemiological link
3. Confirmed case
Any person meeting the clinical and laboratory criteria for case confirmation
*Serological results should be interpreted according to the vaccination status and previous exposure to other flaviviral infections. Confirmed cases in such situations should be validated by serum neutralization assay or other equivalent assays.
As ECDC case definition and reporting have not been universally implemented around the globe or even throughout Europe, data on the burden of disease from different countries are difficult to compare. Even if clear case definitions are provided and routinely implemented by local authorities, differences between countries exist regarding the classification of clinical diseases associated with TBEV infections. For example, Austria reports only “serologically proven hospitalized cases,” whereas the Czech Republic reports any case with “clinical and laboratory signs of aseptic meningitis / meningo-encephalitis, not necessarily associated with hospitalization and Germany reports all diagnosed (serology, virus detection) human infections, irrespective of their clinical manifestation.”8
In addition to the use of different case definitions and case classifications, there is a lack of implementation of routine diagnostics in any patients with CNS infection . This is exemplified by the Polish experience: between 2004 and 2008, only 39% of the country’s hospitals had access to TBEV-serology. Therefore, a pilot project of enhanced surveillance for TBE was implemented in 2009. Routinely testing for TBE in patients with signs of meningitis or encephalitis in the entire country doubled case numbers in 2009 compared to previous years, moreover, and additional 38 endemic districts were identified. Seven of the “new” endemic districts were located far away from previously known endemic foci, most notably in the northwest of the country.9
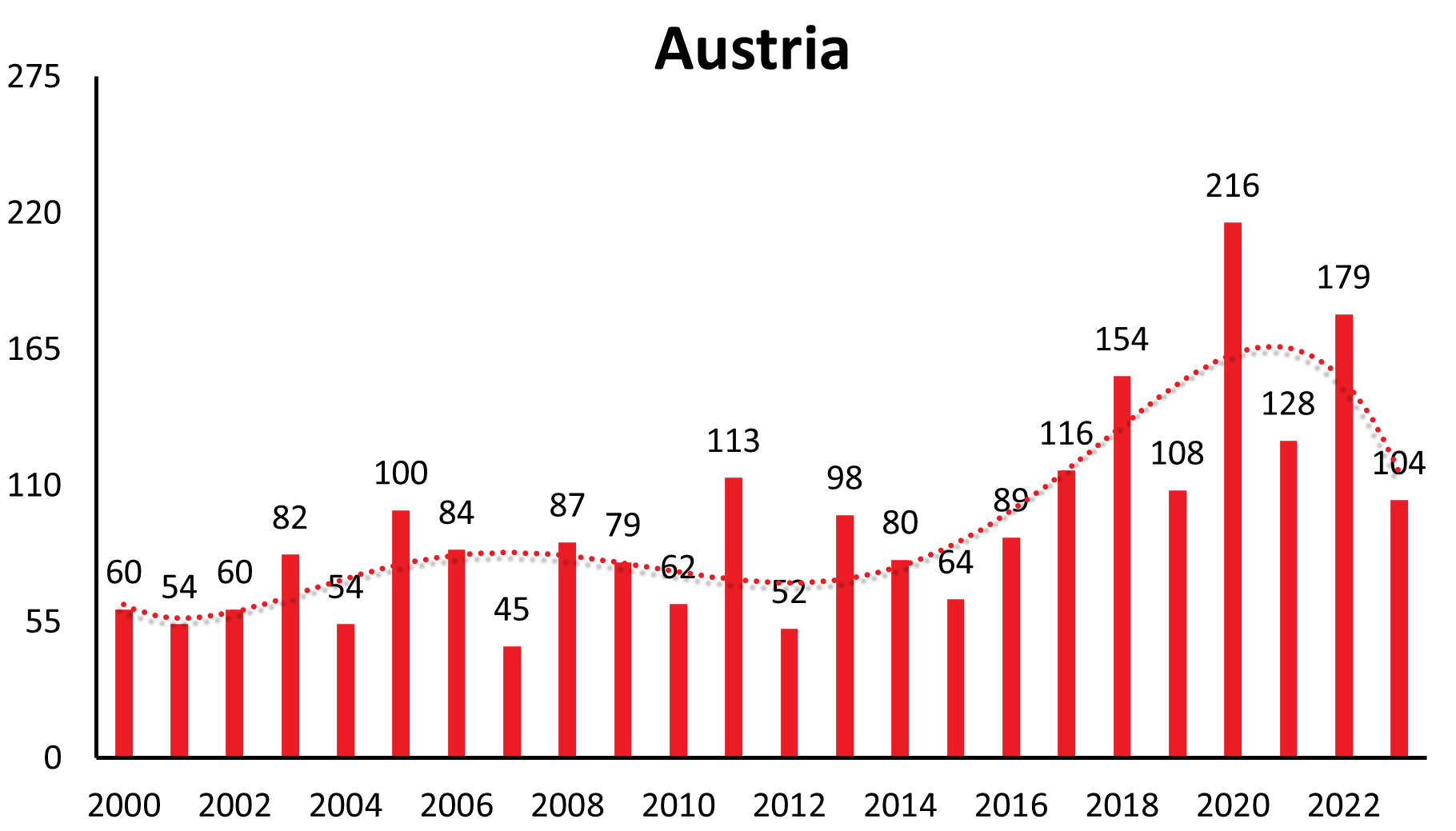
Finally, vaccine uptake substantially modifies the number of cases in a TBE risk area, as exemplified again by Austria, where in the last decade fewer than 100 cases are reported annually while this number, however, had been up to 700 cases annually before the introduction of a vaccination program. The 7-fold difference is easily explained by the about 84% vaccine uptake in Austria. Neighboring countries with lower vaccine uptake continue to have increasing TBE case numbers.10
It should be noted, that there are many “fever only” TBE virus infections without ZNS symptoms not being captured by the ECDC definition.11
1.2. Burden of disease: Incidence and trends
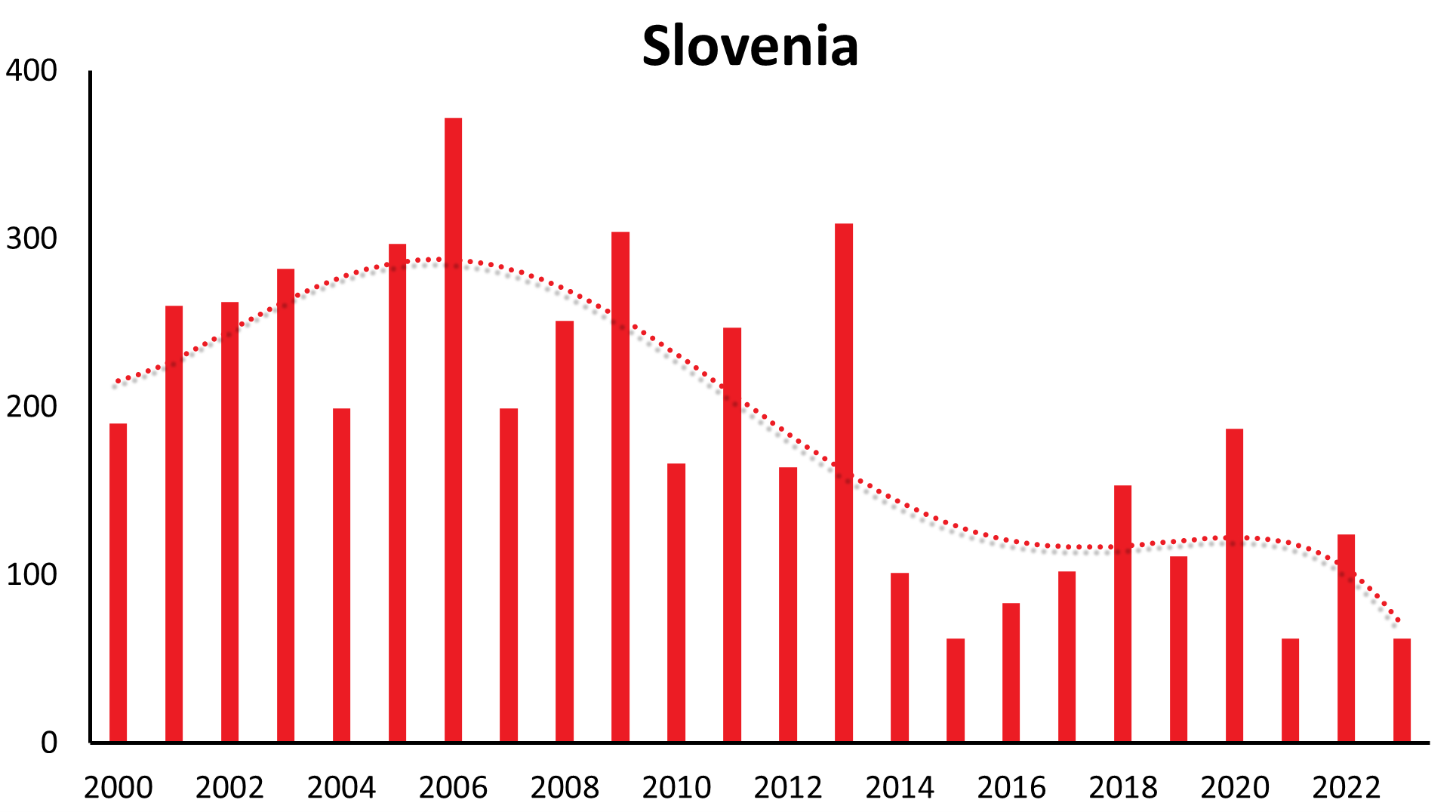
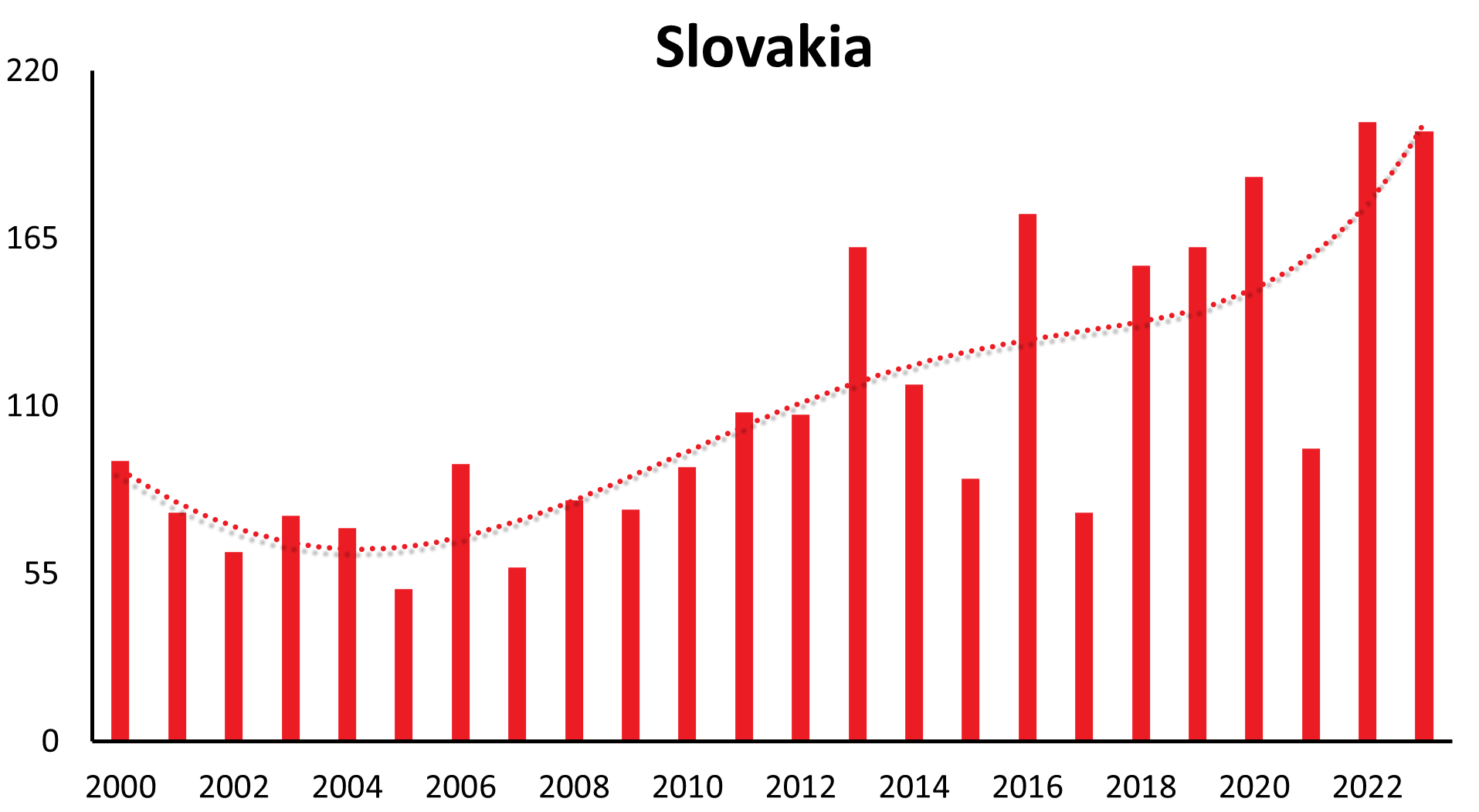
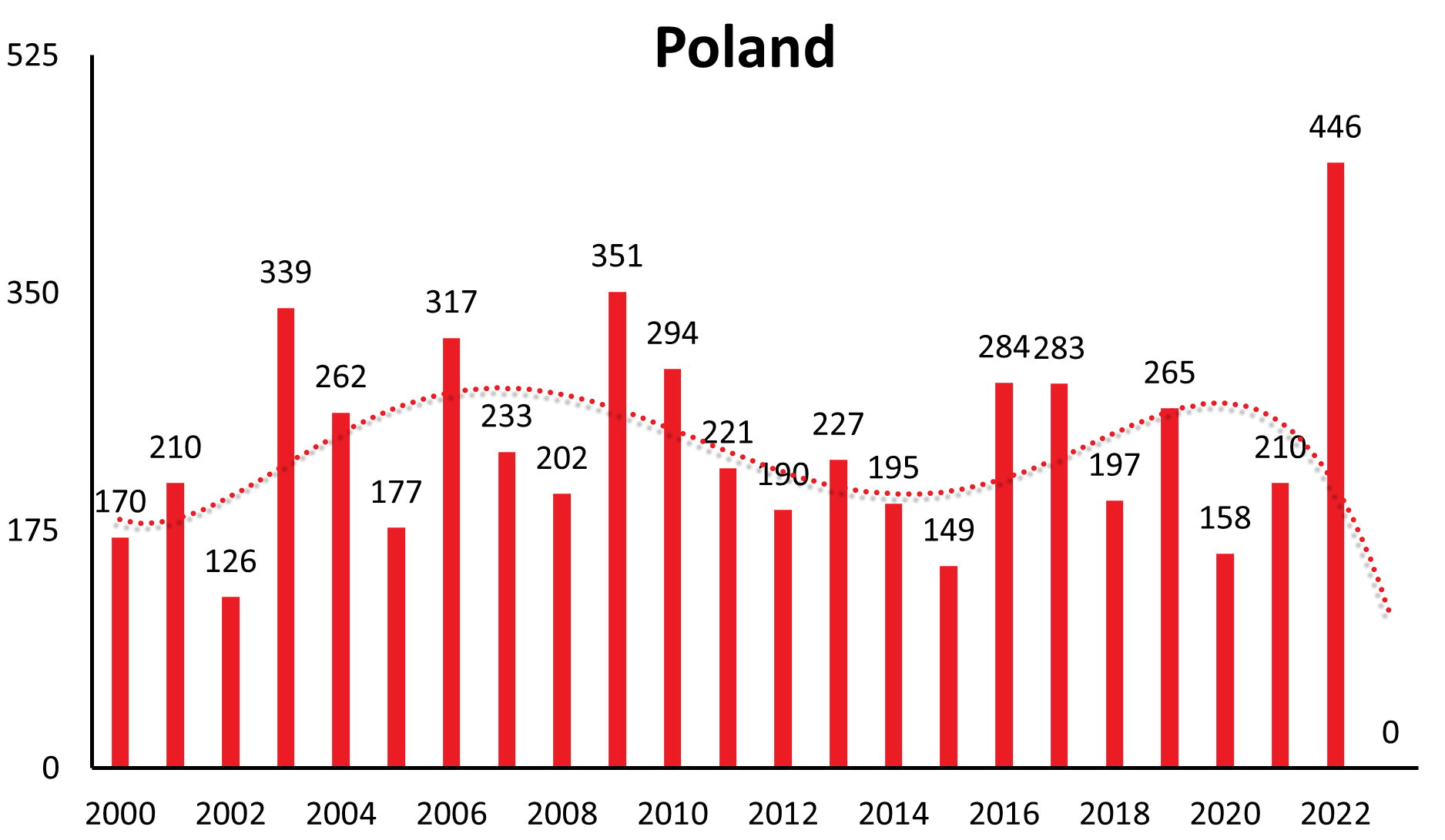
A characteristic feature of TBE is that the incidence of the disease in risk areas can vary significantly from year to year. However, in addition to short-term fluctuations, there are also longer-range undulations of incidence rates recognizable in many countries (refer to Table 2 for TBE cases by country and year).
Table 2: TBE cases by year and country
(source: data provided by the authors of Chapter 13, among others available upon request)
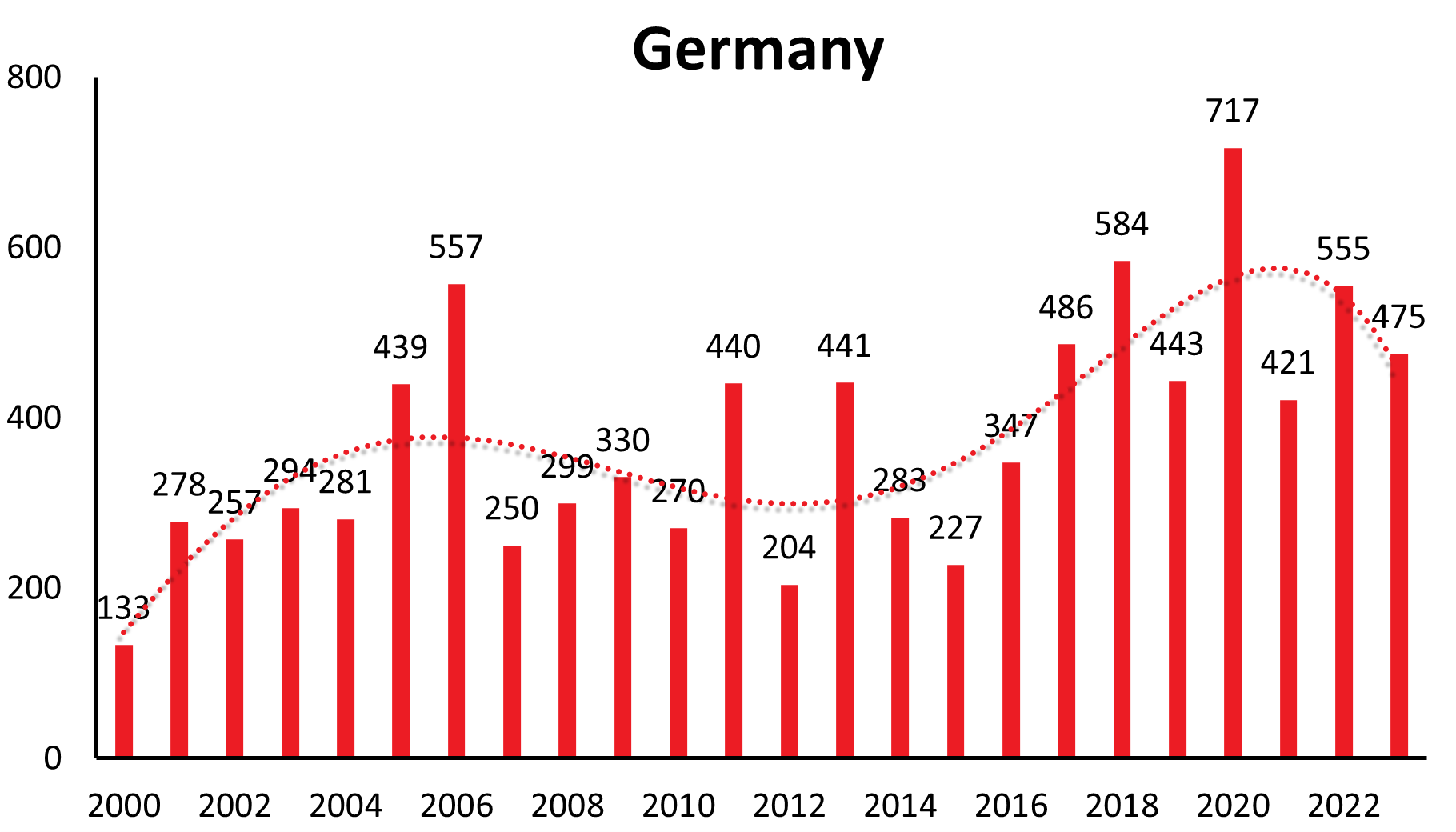
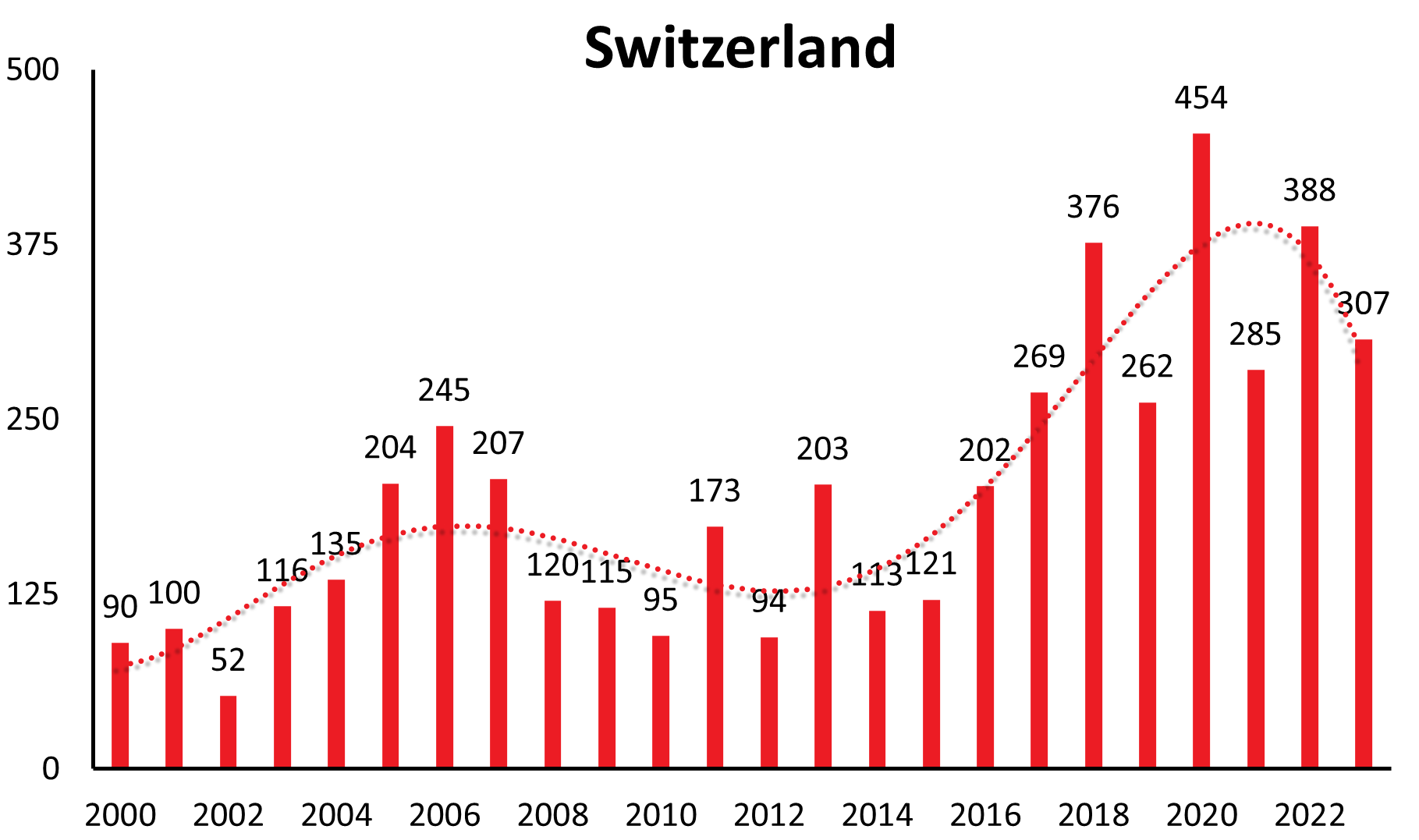
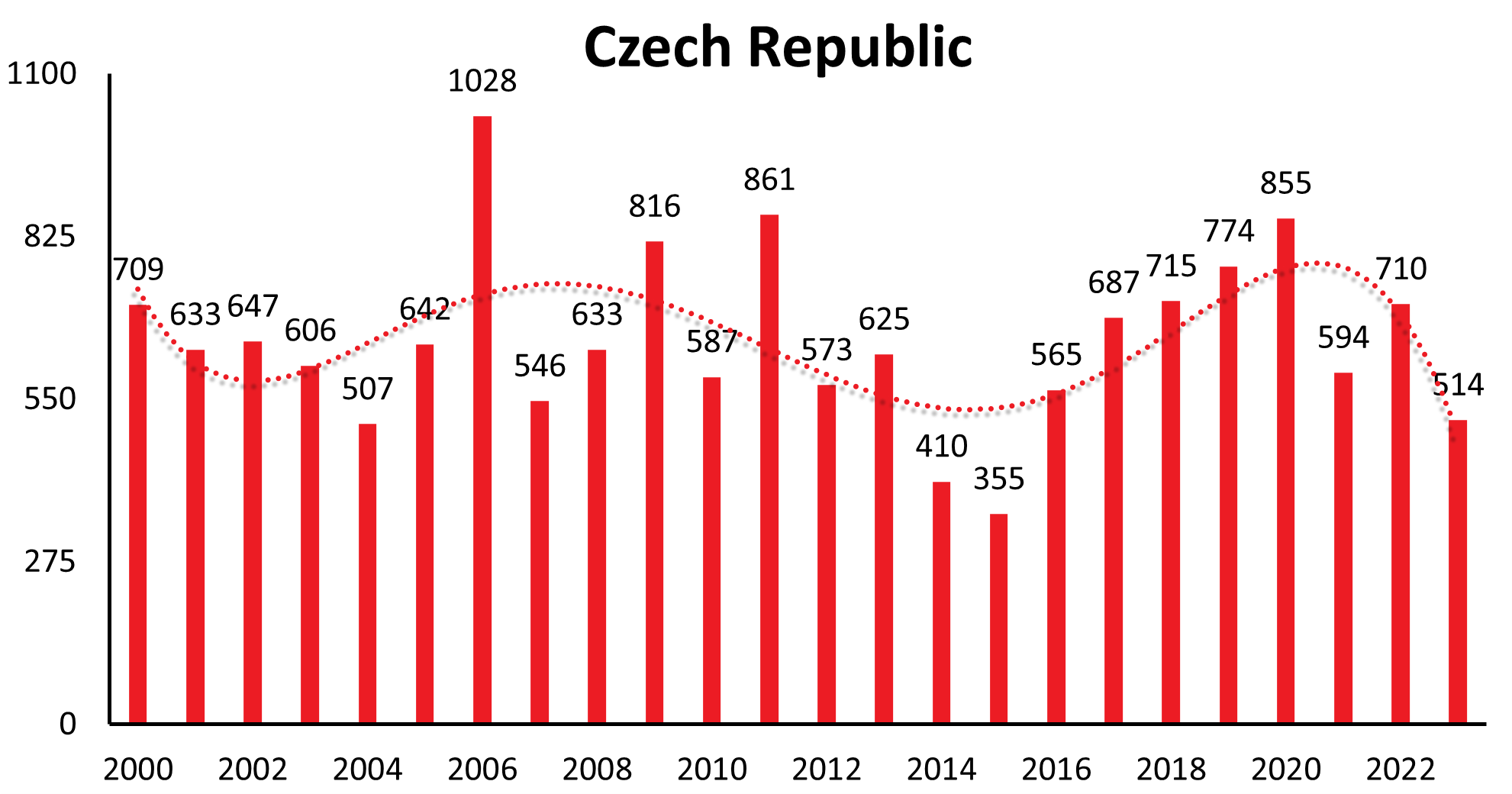
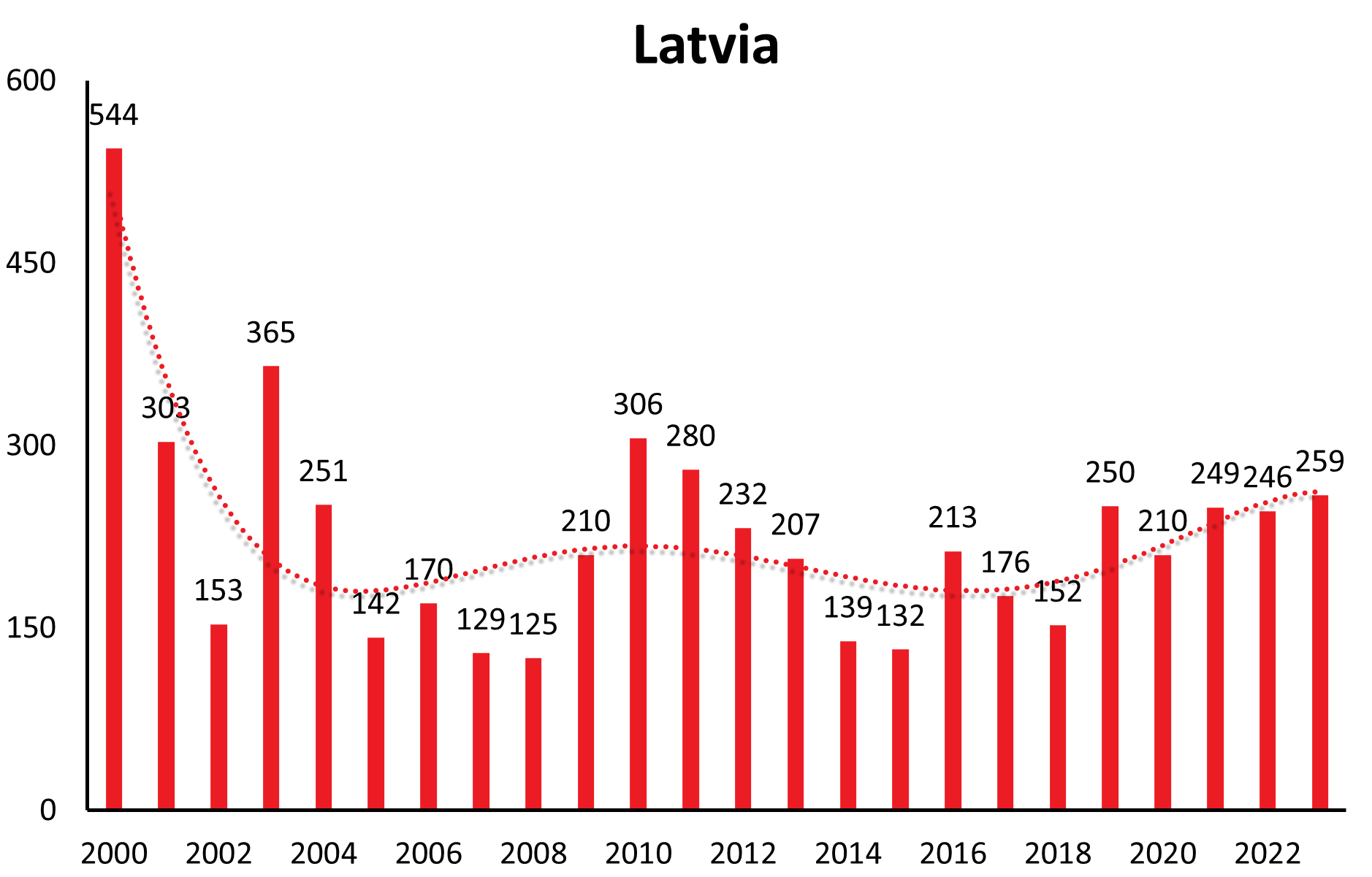
| Country | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 128 | 84 | 102 | 178 | 109 | 128 | 99 | 62 | 41 | 60 | 54 | 60 | 82 | 54 | 100 | 84 | 45 | 87 | 79 | 62 | 113 | 52 | 98 | 80 | 64 | 89 | 116 | 154 | 108 | 216 | 128 | 179 | 104 |
| Belarus | 23 | 61 | 18 | 53 | 44 | 46 | 108 | 82 | 66 | 88 | 91 | 108 | 122 | 109 | 119 | 77 | 141 | 142 | 135 | 171 | 108 | 108 | No data | No data | |||||||||
| Belgium | 2 | 3 | 1 | 1 | 3 | 2 | 0 | 3 | 2 | 2 | 2 | ||||||||||||||||||||||
| Bosnia and Hercegovina | 1 | 2 | 5 | 0 | N/A | N/A | N/A | ||||||||||||||||||||||||||
| Bulgaria | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | ||||||||||||||||||
| China | No data | ||||||||||||||||||||||||||||||||
| Croatia7 | 60 | 27 | 76 | 87 | 59 | 57 | 25 | 24 | 26 | 18 | 27 | 30 | 36 | 38 | 28 | 20 | 12 | 20 | 44 | 36 | 26 | 45 | 44 | 42 | 25 | 6 | 10 | 24 | 14 | 15 | 4 | 23 | 9 |
| Czech Republic | 356 | 337 | 618 | 619 | 727 | 571 | 412 | 422 | 490 | 709 | 633 | 647 | 606 | 507 | 642 | 1028 | 546 | 633 | 816 | 589 | 861 | 573 | 625 | 410 | 355 | 565 | 687 | 715 | 774 | 855 | 594 | 710 | 514 |
| Denmark | 2 | 2 | 3 | 4 | 3 | 3 | 1 | 4 | 8 | 2 | 2 | 1 | 2 | 2 | 4 | 1 | 1 | 3 | 1 | 1 | 1 | 0 | 4 | 5 | 5 | 7 | 5 | 12 | |||||
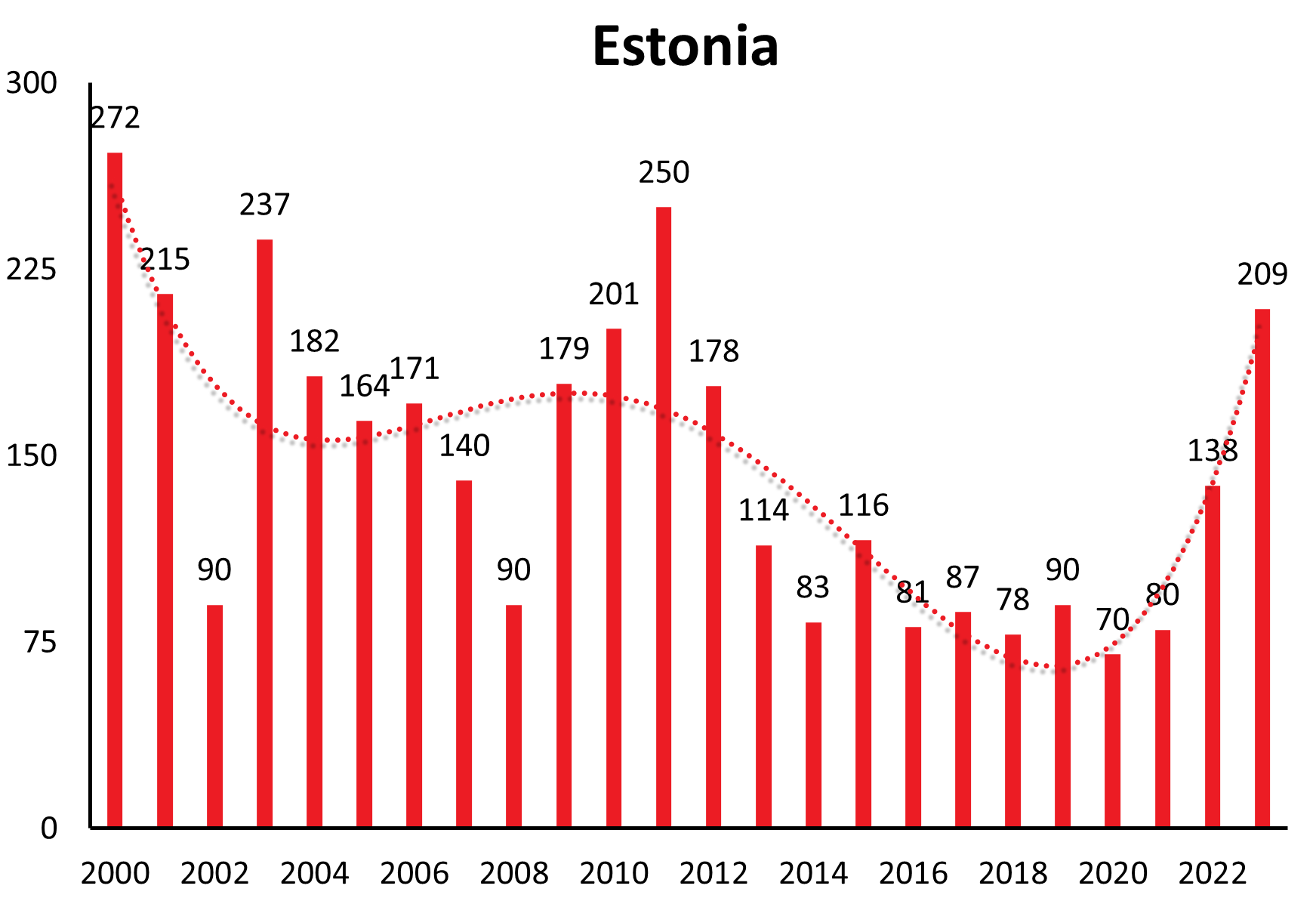
| Estonia | 68 | 163 | 166 | 177 | 175 | 177 | 404 | 387 | 185 | 272 | 215 | 90 | 237 | 182 | 164 | 171 | 140 | 90 | 179 | 201 | 250 | 178 | 113 | 84 | 116 | 81 | 87 | 85 | 83 | 70 | 80 | 138 | 209 |
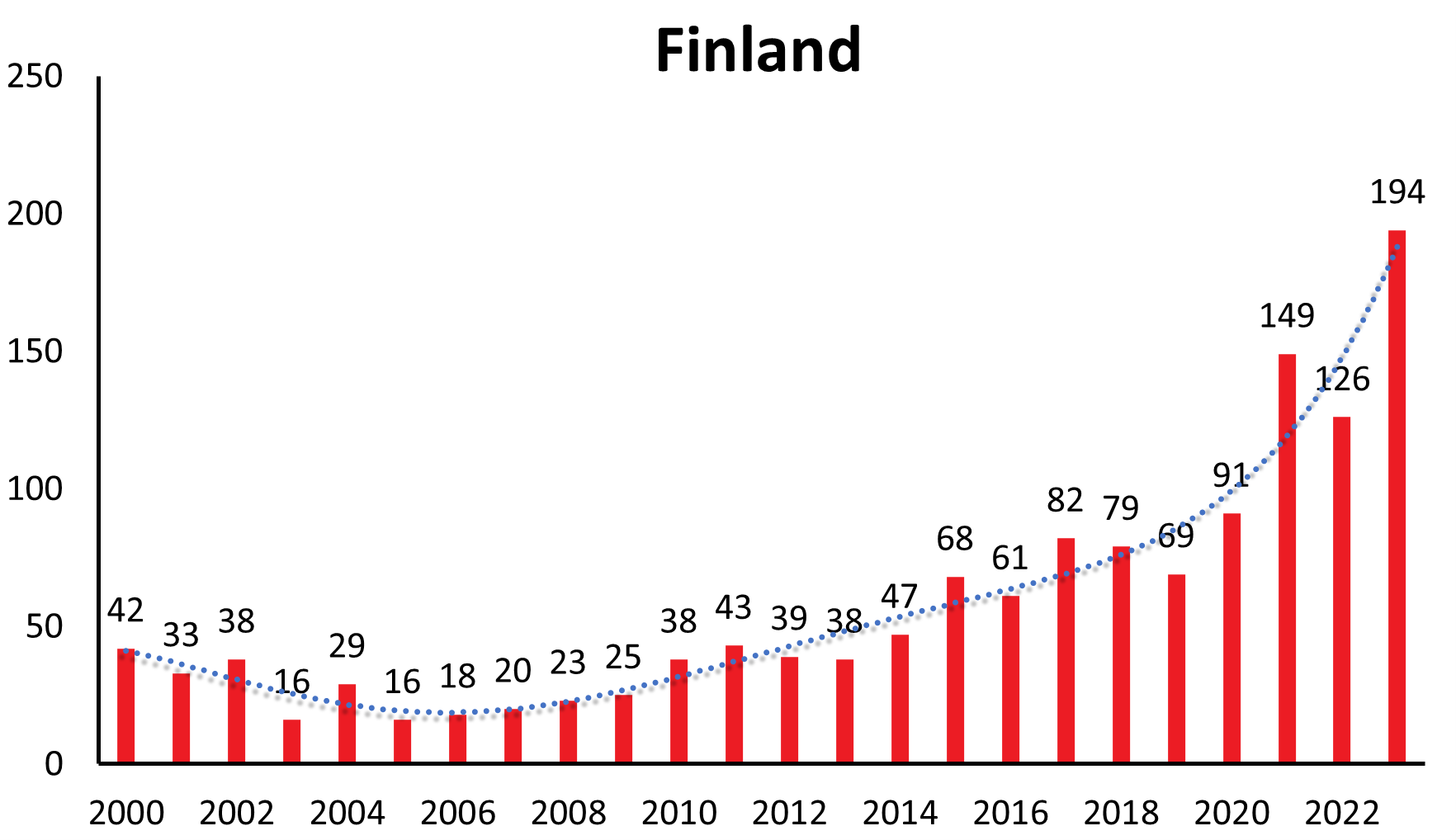
| Finland | 14 | 25 | 16 | 23 | 8 | 19 | 16 | 12 | 42 | 33 | 38 | 16 | 29 | 16 | 18 | 20 | 23 | 25 | 38 | 43 | 39 | 38 | 47 | 68 | 61 | 82 | 79 | 69 | 91 | 148 | 126 | 194 | |
| France | 1 | 1 | 4 | 3 | 4 | 1 | 2 | 2 | 5 | 5 | 8 | 4 | 3 | 8 | 4 | 10 | 6 | 6 | 2 | 3 | 8 | 4 | 4 | 10 | 11 | 29 | 18 | 24 | 24 | 68 | 31 | 31 | No data |
| Germany | 44 | 142 | 118 | 306 | 226 | 114 | 211 | 148 | 115 | 133 | 255 | 239 | 277 | 274 | 432 | 544 | 239 | 289 | 313 | 260 | 424 | 195 | 420 | 264 | 221 | 353 | 485 | 582 | 443 | 717 | 421 | 555 | 475 |
| Hungary | 299 | 190 | 339 | 264 | 234 | 246 | 102 | 74 | 69 | 54 | 55 | 80 | 114 | 89 | 54 | 57 | 63 | 55 | 70 | 50 | 43 | 44 | 53 | 31 | 24 | 19 | 16 | 32 | 18 | 18 | 6 | 29 | 24 |
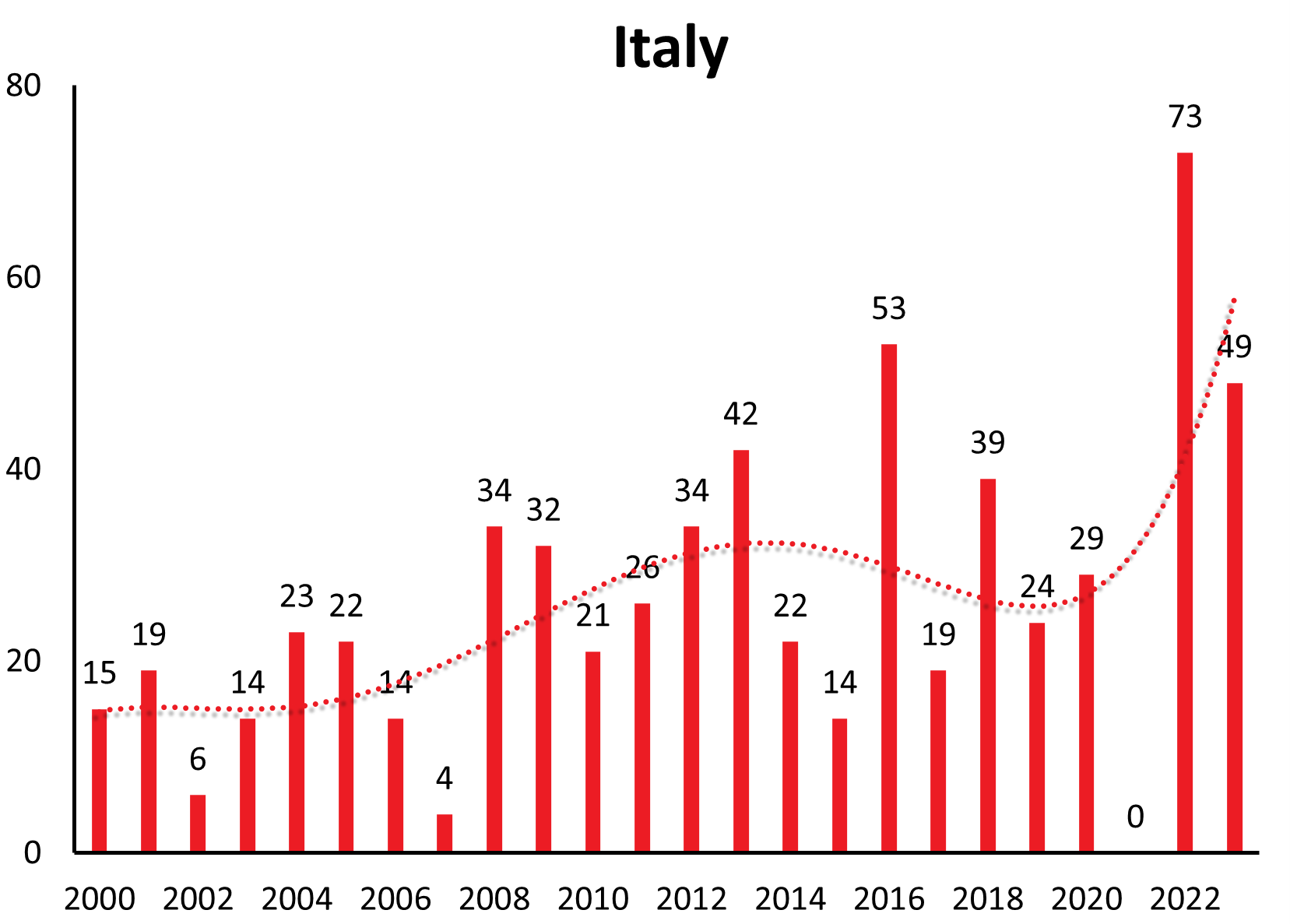
| Italy | 0 | 2 | 2 | 8 | 6 | 8 | 8 | 11 | 5 | 12 | 24 | 9 | 17 | 32 | 25 | 44 | 21 | 26 | 34 | 21 | 26 | 34 | 42 | 22 | 14 | 53 | 24 | 40 | 37 | 55 | 14 | 73 | 49 |
| Japan | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||
| Kazakhstan | 20 | 19 | 12 | 17 | 22 | 30 | 43 | 38 | 60 | 44 | 35 | 55 | 30 | 50 | 49 | 33 | 32 | 34 | 49 | 30 | 40 | 33 | 27 | 28 | 49 | 48 | 34 | 46 | 35 | 31 | 24 | 32 | 24 |
| Kirgistan | N/A | N/A | N/A | ||||||||||||||||||||||||||||||
| Latvia | 227 | 287 | 791 | 1366 | 1341 | 736 | 874 | 1029 | 350 | 544 | 303 | 153 | 365 | 251 | 142 | 170 | 129 | 125 | 210 | 306 | 280 | 232 | 207 | 139 | 132 | 213 | 176 | 152 | 211 | 210 | 249 | 246 | 259 |
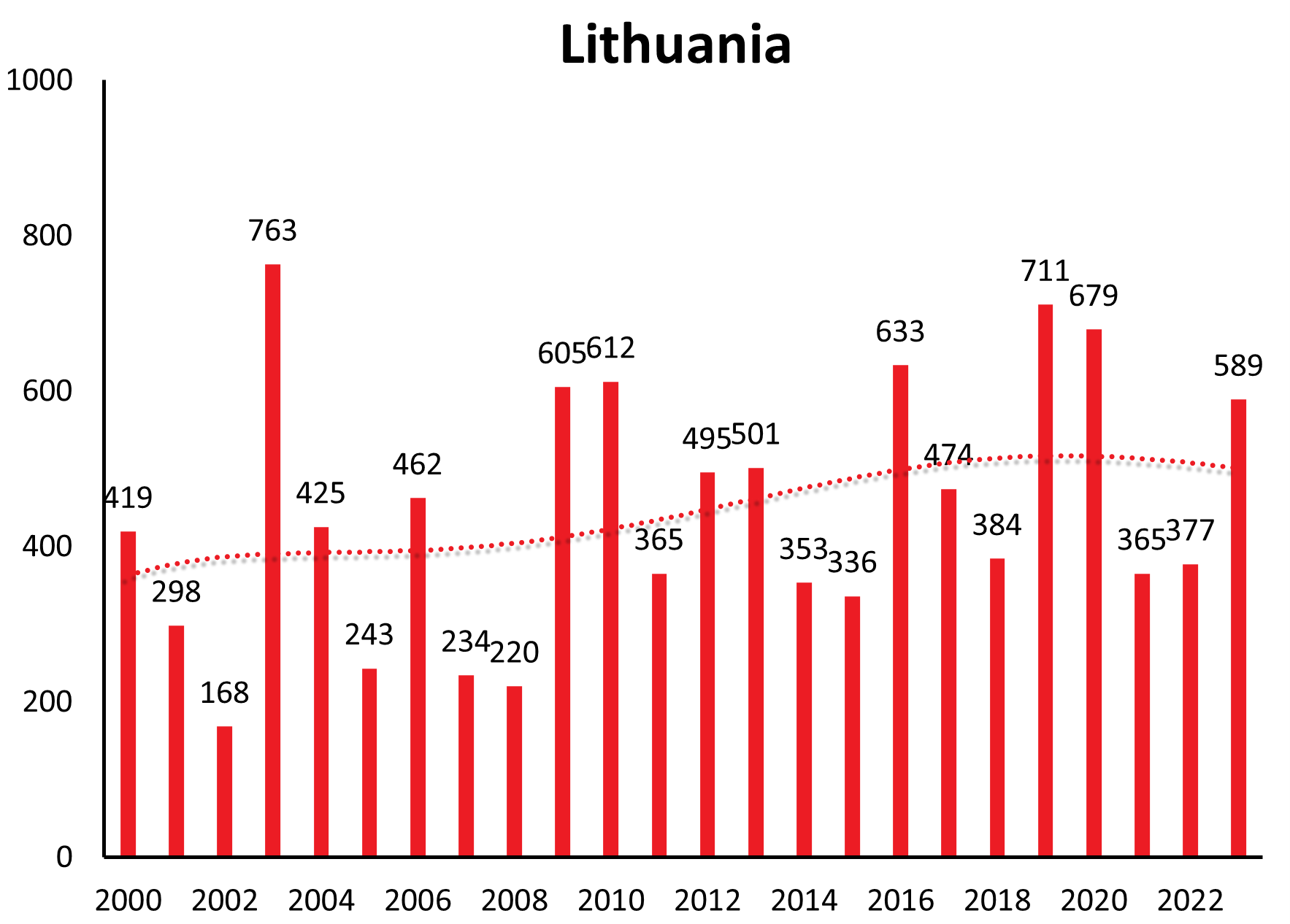
| Lithuania | 14 | 17 | 198 | 284 | 427 | 310 | 645 | 548 | 171 | 419 | 298 | 168 | 763 | 425 | 243 | 462 | 234 | 220 | 605 | 612 | 365 | 495 | 501 | 353 | 336 | 633 | 474 | 384 | 711 | 679 | 365 | 377 | 589 |
| Moldova | 0 | No data | No data | No data | |||||||||||||||||||||||||||||
| Mongolia | 5 | 6 | 52 | 12 | 8 | 9 | 13 | 6 | 15 | 7 | 40 | 52 | 62 | 32 | 19 | 20 | 5 | 8 | 34 | ||||||||||||||
| Netherlands | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 5 | 2 | 2 | 5 | ||||||||||||||||||||||
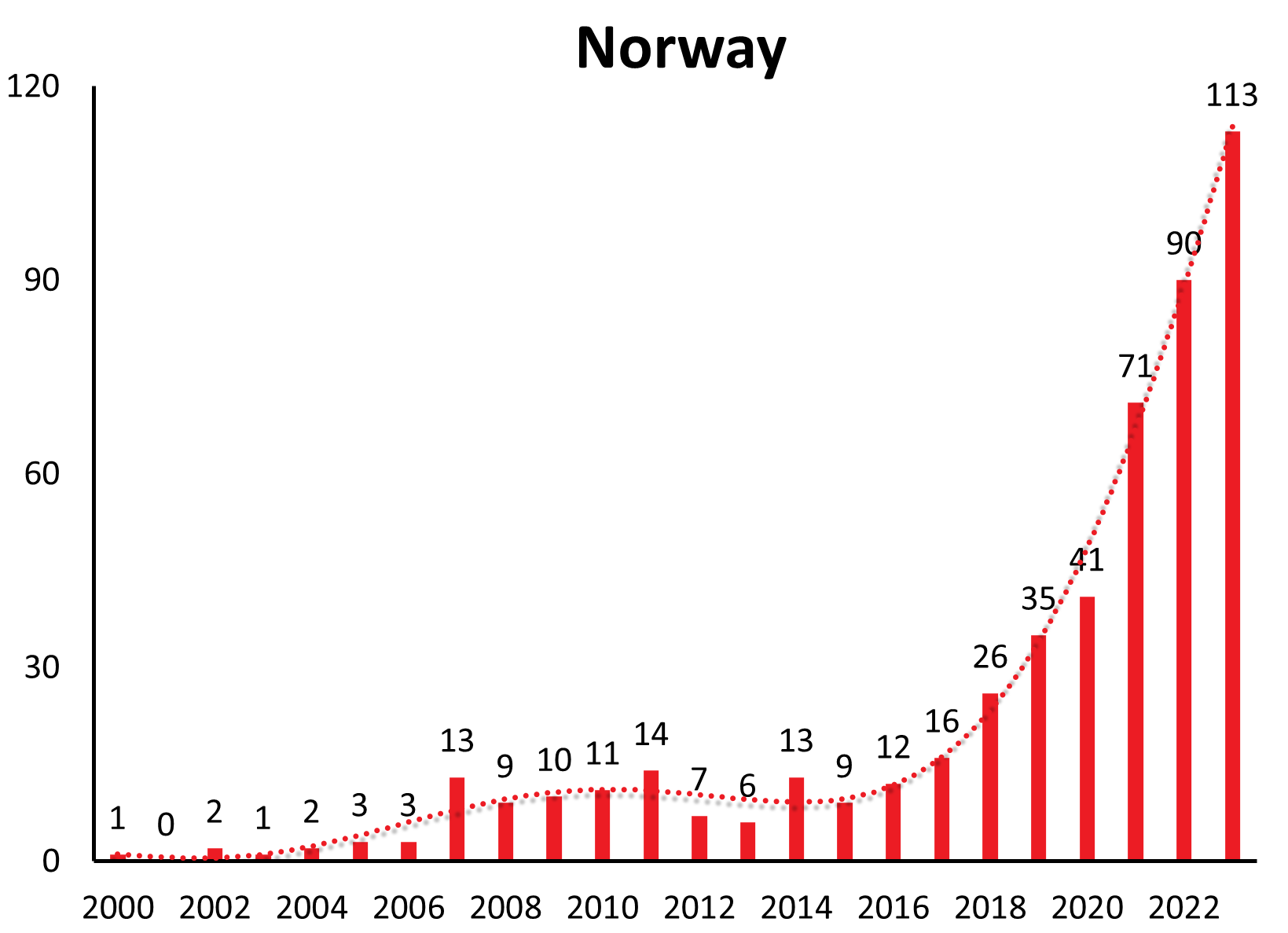
| Norway | 1 | 1 | 1 | 0 | 2 | 1 | 4 | 4 | 5 | 13 | 11 | 10 | 11 | 14 | 7 | 6 | 13 | 9 | 12 | 16 | 26 | 35 | 41 | 71 | 90 | 113 | |||||||
| Poland | 4 | 8 | 241 | 181 | 267 | 259 | 201 | 208 | 208 | 170 | 210 | 126 | 339 | 262 | 177 | 317 | 233 | 202 | 351 | 294 | 221 | 190 | 227 | 195 | 149 | 284 | 283 | 197 | 265 | 158 | 210 | 446 | 659 |
| Romania | 8 | 4 | 3 | 3 | 3 | 1 | - | 0 | No data | No data | No data | ||||||||||||||||||||||
| Russia | 5194 | 6239 | 7571 | 5640 | 5935 | 10371 | 6804 | 7531 | 10011 | 6010 | 6569 | 5231 | 4773 | 4178 | 4593 | 3433 | 3142 | 3140 | 3141 | 3094 | 3533 | 2716 | 2236 | 1978 | 2304 | 2035 | 1934 | 1727 | 1781 | 989 | 1015 | 1969 | N/A |
| Serbia | 1 | 6 | 1 | 4 | 4 | 1 | 5 | 13 | 0 | 0 | 1 | 1 | |||||||||||||||||||||
| Slovakia | 24 | 16 | 51 | 60 | 89 | 82 | 76 | 54 | 63 | 92 | 75 | 62 | 74 | 70 | 50 | 91 | 57 | 79 | 76 | 90 | 108 | 107 | 162 | 117 | 88 | 174 | 75 | 156 | 161 | 185 | 96 | 203 | 200 |
| Slovenia | 118 | 80 | 197 | 531 | 157 | 406 | 274 | 137 | 150 | 196 | 260 | 262 | 282 | 199 | 297 | 372 | 199 | 251 | 304 | 166 | 247 | 164 | 309 | 100 | 62 | 83 | 102 | 153 | 111 | 187 | 62 | 124 | 62 |
| South Korea | 0 | N/A | No data | N/A | |||||||||||||||||||||||||||||
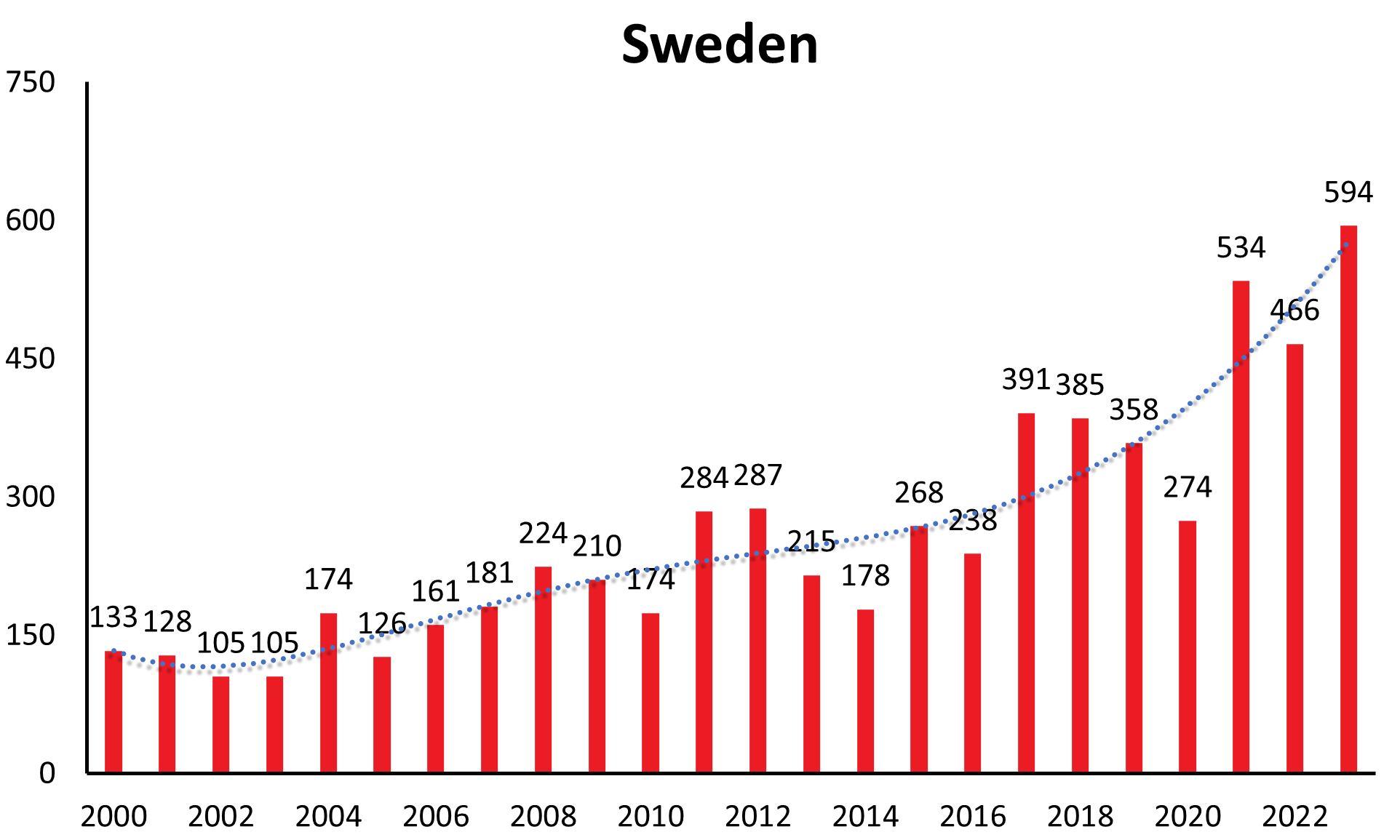
| Sweden | 68 | 84 | 48 | 116 | 67 | 45 | 74 | 65 | 53 | 133 | 128 | 104 | 101 | 174 | 126 | 161 | 181 | 224 | 210 | 174 | 284 | 287 | 209 | 178 | 268 | 238 | 391 | 385 | 358 | 274 | 534 | 466 | 594 |
| Switzerland | 37 | 66 | 44 | 97 | 60 | 62 | 123 | 68 | 112 | 89 | 96 | 52 | 114 | 131 | 204 | 238 | 105 | 119 | 112 | 96 | 170 | 96 | 202 | 108 | 122 | 200 | 273 | 369 | 259 | 448 | 285 | 388 | 307 |
| Tunesia | 0 | No data | No data | ||||||||||||||||||||||||||||||
| Ukraine | 12 | 28 | 4 | 8 | 7 | 4 | 7 | 8 | 3 | 10 | 3 | 3 | 6 | 3 | 6 | 4 | 5 | 2 | 2 | No data | No data | No data | |||||||||||
| United Kingdom | 2 | 0 | 2 | No data |
In Estonia for example, a country with one of the highest overall TBE incidences in Europe case numbers in the years 2005 – 2017 fluctuated between 6.2 and 18.6 with a mean incidence between 5.2 and 52.8 (see Chapter 13, Estonia).
These longer-range undulations are well recognized and in synchrony in a time interval of 12-15 years in countries like Germany, Czech Republic, Slovakia, Switzerland, Austria (see Figure 1a), and similarly in Poland and Slovenia. The long-term trend, however, shows an increase in Germany, Austria, Slovakia, Switzerland and Poland, a constant trend the last 22 years in the Czech Republic and decreasing in Slovenia.
Countries like Lithuania, Estonia, Latvia show a similar long-range undulation of about 12-15 years, time-wise incongruous to the central European countries. Trends in case numbers however are constant over time (Lithuania, Latvia) or even decreasing long-term (Estonia) (see Figure 1b).
Disease numbers in Sweden, Finland, Norway and even Italy have shown a substantial and continuous increase in the last couple of years (see Figure 1c). In Sweden there is an increase reported from approximately 1.9/100,000 inhabitants in 2010 to 5.1/100,000 inhabitants in 2021.12 However, those countries do not have the same long-range awareness and screening as the countries mentioned above. So this observed increase may at least in part be explained by an increasing awareness and surveillance in the respective country. (e.g. Sweden13)
But also new countries, until recently regarded TBE-virus free, have been identified in the last decade as areas where TBEV circulates.
Since 2016, the Netherland,14,15 Belgium16 and the United Kingdom17,18 have reported autochthonous human cases. Recently, TBEV has even been detected in ticks collected in North Africa, in Tunisia (see country chapter). These findings illustrate that increased awareness and forced investigations to detect TBEV can lead to identification of new TBEV endemic areas and “artificially” increase cases numbers.
In recent years, new TBE foci have been reported from altitudes up to 2100 meters above sea level.19-22 New endemic zones in previously unaffected alpine regions in western Austria23 and in Switzerland were established, and a first report of TBEV being detected at locations in Norway up to more than 65°N latitude was published 2018.24
Various factors may explain all these findings, at least in part: social factors (socio-political changes with changes in human behavior, duration, and type of leisure-time activities), ecological factors (e.g., effects of climate changes on the tick population and change in availability of tick host species, new flight routes of migrating birds which may lead to importation of TBE virus infected ticks into areas which have so far been free of TBE virus), and/or technological factors (advanced diagnostics, increased medical awareness).
There is increasing research interest in habitat suitability modeling to define universal environmental characteristics of TBEV foci, to predict suitable conditions where potentially human TBEV infections may occur.25-27
Certainly, reporting of TBE cases has improved substantially over the years, and TBE is now a notifiable disease in the EU. In the end, all factors mentioned above play an “interactive role” resulting in complex interactions that may explain the observed changes in TBE epidemiology. But still, TBE surveillance in Europe is in many countries more sporadic than systematic, and TBE cases are likely underreported.2,28
2. TBE risk areas
2.1. Risk area definition
The TBE virus is restricted to specific endemic regions, and various procedures can be used to assess if and where TBE virus is circulating.
- One of the most common methods used are antibody-prevalence studies in sera from humans or animals using ELISAs or indirect immunofluorescence tests which have the advantage that a high number of sera can be tested in relatively short time.
- However, cross-reactivity with other flaviviruses can be misleading, and therefore, confirmation by neutralising tests are of upmost importance.
- Another approach, but less often used, is the detection of TBEV-specific genomic sequences in ticks or in samples of milk from infected hosts like sheep, goats or cows.
For many countries in Eurasia, which are classified as TBE risk areas and are part of the TBE belt, this assessment is based on the sum of different documented evidence. Interpreting the results of such investigations and the definition of such risk areas is tricky and may be influenced by a number of factors:
- Very often the exact place of human TBE infection cannot be determined with certainty
- Epidemiology of TBE is the result of a complex interaction between reservoir animals, birds, ticks, vegetation, climate, weather.
- Human case numbers are to be interpreted with care. Behavior may change from time to time, and population density may be different in different regions of the world (see following country chapters for details). Finland for example is the eighth-largest country in Europe and the most sparsely populated country in the European Union (Population density is 18 inhabitants per square kilometer. This is the third-lowest population density of any European country). The majority of the population lives in the central and southern parts of the country. However, according to monitoring data for 2015–2019, the calculated incidence of tick-borne encephalitis in 2019 is as high as 53 per 100.000 inhabitants in the municipality of Pargas, 42 in Simo, 20 in Kustavi, and it is 30 on the island of Åland. Recommendations per municipality are based on human incidence numbers exclusively and do not consider those many municipalities where there are only few people living.
- Environmental variables change annually resulting in great annual differences in case numbers, demonstrated in the country chapters as well as in Table 2. For instance, in some highly endemic areas, TBEV prevalence in ticks reaches 20– 40%, but in other areas it can be as low as 0.1–0.5%31 (see Chapter 11)
- A high local vaccine uptake may result in a low disease incidence, whereas the incidence in the unvaccinated (e.g., a traveler) may be much higher than the reported risk in the local population indicates. This is relevant information for travelers.
- TBEV-infected ticks are typically found in microfoci, i.e. the virus is often detectable in small areas only, whereas the surrounding areas are TBEV-free.
To date there is no commonly accepted definition to characterize “TBE risk areas”. Most definitions and consequently vaccination recommendations (even from the WHO) so far are based on the human TBE incidence numbers in a given area.
A more holistic proposal was presented by the ECDC for assessing the risk for arbovirus infections in general.29
- The key point from this is that “… any area where the chances of transmission of an arthropod-borne disease to humans are higher than nil is a risk area.” This definition is compelling as it refrains from requiring any specific level of risk (which can be small or large), like incidence data, which vary from year to year even for the same region.
- The authors then graded risk areas as follows29:
- A predisposed area is a risk area where existing conditions might enable the transmission of an arthropod-borne disease to humans, but the respective pathogen has not been detected. This may result from the fact that now surveillance for the TBEV had been accomplished to date.An imperiled area is a risk area with no human cases detected, but where the pathogen has been detected in vectors, or transmission of the pathogen to animals or humans has been detected indirectly (by serology, e.g. if routine testing is not available). An affected area is a risk area, where human TBE disease cases have occurred either sporadically or in a timewise restricted matter.
- An endemic area is a risk area where recurrent transmission of TBE to humans is taking place over several seasonal cycles.
In order to assign an arbovirus-risk based on the ECDC definition29 an area must be accurately determined geographically and by biological and epidemiological findings (surveillance of human and animal cases, field investigation etc.) in order to avoid misunderstandings and imprecision.
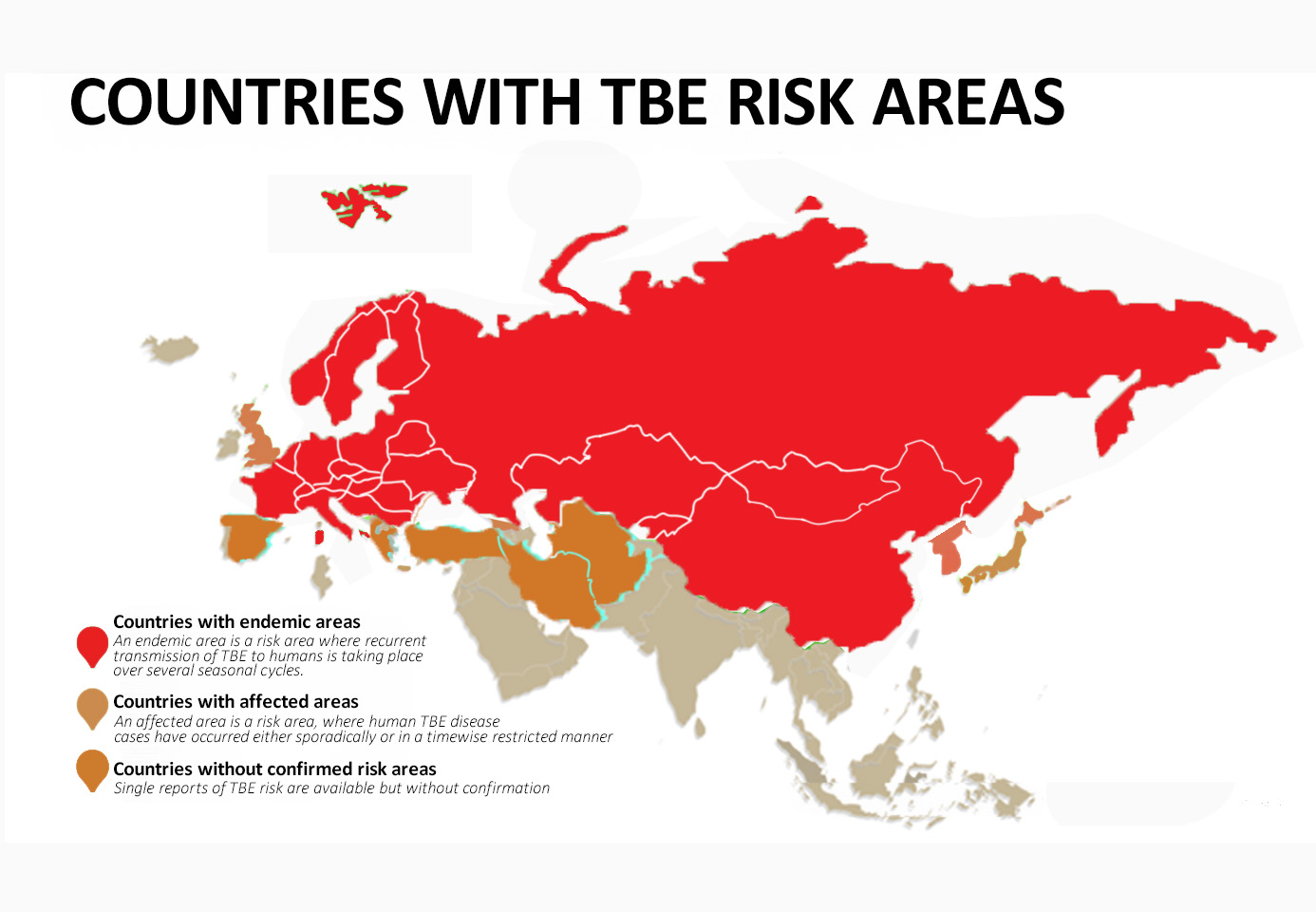
This however is NOT the case with TBE, as the quality of surveillance and reporting is significantly different among countries and data cannot be simply compared. Therefore, the ECDC classification is a way to grade available evidence by the time of evaluation. However, as noted above, the epidemiology of TBE is a “moving target”, the process of unequivocal classification of a country as TBE risk area and the decision on vaccination recommendation is a stepwise process and can take many years. Countries with reasonable evidence for risk area assessment (see Figure 2) are discussed in the respective country chapters (see below). For some countries, preliminary data are available regarding the prevalence of TBE virus which do not yet allow a risk area assessment. (see below paragraph 2.3.)
Figure 2: Countries with TBE risk areas
(as discussed in the respective country chapters and their status – endemic – affected – without confirmed risk area)
2.2. TBEV subtype and vector distribution
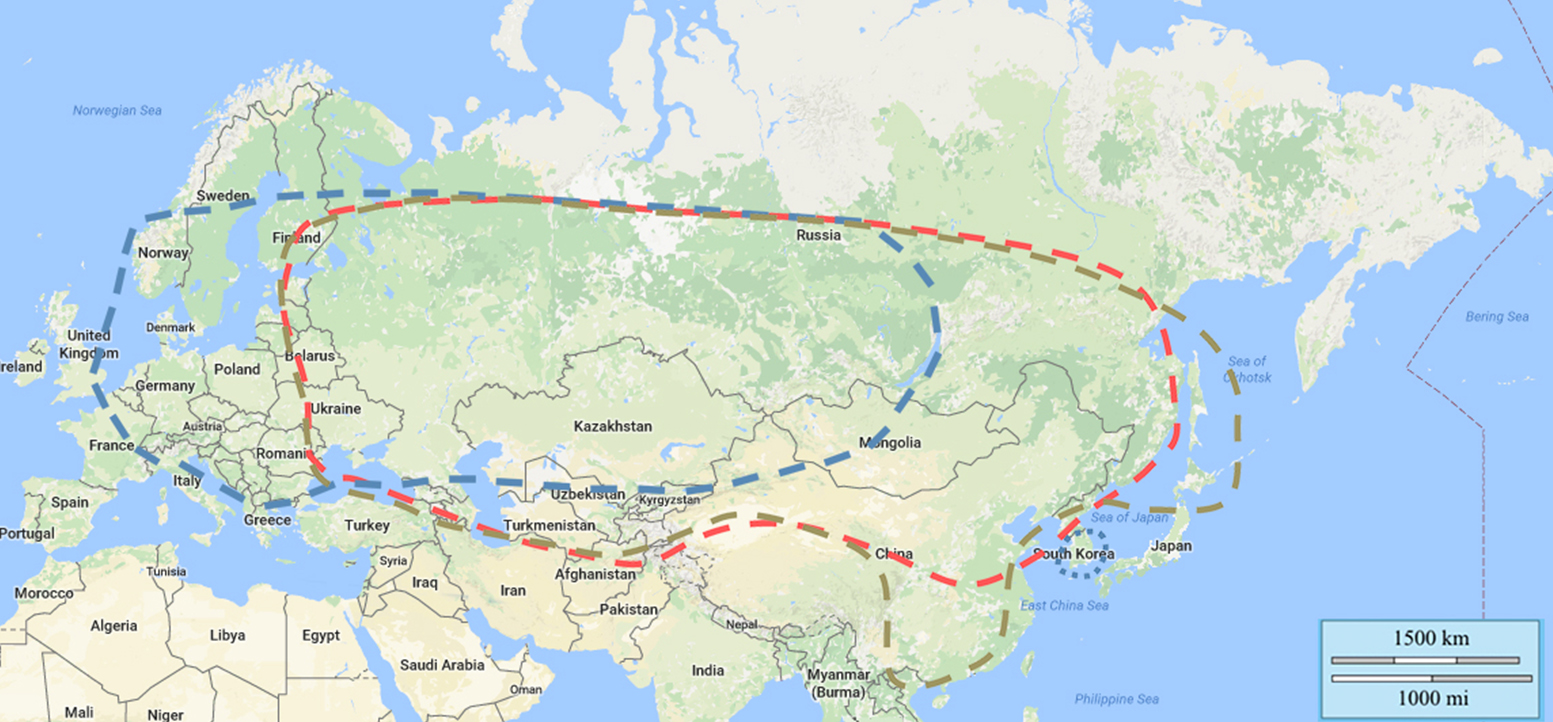
Three main TBEV subtypes have been described based on their distribution pattern and sequence similarity (see Figure 3): the European subtype virus (previously CEE virus, Central European encephalitis virus; TBEV-EU), the Far Eastern subtype (previously RSSE virus; TBEV-FE), and the Siberian subtype (previously west Siberian virus; TBEV-Sib). In addition to the 3 primary TBEV subtypes, there is a fourth accepted subtype, designated as Baikalian subtype (TBEV-BKL) with the prototype strain “886-84”. Recently, two additional lineages have been described as possible TBEV subtypes, namely the “strain 178-79”, and the Himalayan subtype (TBEV-HIM)30 (details see chapter 11). So far, it is unclear whether the recently detected strain “Sallandse” from The Netherlands forms its own subtype or belongs to the European subtype.
Figure 3: Distribution of TBEV subtypes by country
Click the image above to enlarge
Distribution of TBEV subtypes:
| TBEV-Eu | dotted blue line: prevails Europe, virus isolates in Siberia also, most eastern virus isolation Lake Baikal |
| TBEV-Sib | dotted red line: prevails Siberia and Ural region, most western virus isolation Baltics and Moldavia, most eastern virus isolation far eastern region of Russia |
| TBEV-Fe | dotted brown line: prevails far eastern region of Russia, most western virus isolation Baltics and Moldavia, most eastern virus isolation Hokkaido, Japan |
| Islands of unusual TBEV subtype distribution are reported in South Korea (TBEV-EU) | |
TBEV-FE prevails in the regions of far-east Russia, in China, Mongolia and in Japan. TBEV-SIB prevails in eastern and western Siberia, in the Ural and European part of Russian territories. TBEV-EU is predominant in Eastern European countries including Ukraine and in central, western, and northern Europe. However, there is a big overlap in the distribution pattern of the three main subtypes as outlined in fig 3.
TBEV-BLK was found in East Siberia near Lake Baikal and in Northern Mongolia, and TBEV-HIM was recently isolated in wild rodents (Marmota himalayana) in the Qinghai-Tibet Plateau in China.30
The principal vector as well as the reservoir for the TBEV-EU subtype is the tick I. ricinus, whereas TBEV-FE and TBEV-SIB subtypes are transmitted predominantly by I. persulcatus. The ranges of the 2 tick species as well as the TBEV subtypes overlap in Estonia, parts of Latvia, Finland, northern Sweden, and the European part of Russia. Interestingly in Finland I. ricinus infected with TBEV‑Sib and I. persulcatus infected with TBEV‑Eu have both been detected.31,32
All 3 main TBEV subtypes have been found in Estonia and Latvia.33,34 From the limited virus isolates available from the Ukraine so far, there is evidence that all TBEV subtypes are present on the Crimean peninsula, too.35,36 The TBEV-SIB has been detected in Bosnia as well.36
TBEV-EU foci have been reported from South Korea, approximately 7000 km away from the European range of the TBEV-EU subtype circulation.37 TBEV strains related to the TBEV-EU subtype were isolated in rodents and humans in eastern and western Siberia as well as in the Ural territory.36,38 TBEV-FE foci have not only been reported from Crimea, about 3000 km away from the known TBEV-FE circulation area39 but also from the Republic of Moldova between 2010 and 2011.40
Geographical circulation of the TBEV subtypes, unusual TBEV subtype foci, and various carrier vectors are well described in more detail in Chapter 2.
2.3. Areas without confirmed TBE risk assessment
As mentioned TBE-virus risk area assessment is a stepwise process and one should consider some core assumptions.
- i) Can tick species which are known as vectors for TBEV be found in the region to be analysed?
- ii) Is the climate of the region and the landscape suitable for these tick vectors?
- iii) How specific are the tests used to detect TBEV? What about cross-reactivity with other flaviviruses especially with those to be expected in the region?
A variety of flaviviruses genetically related to TBEV has been described (without being complete):
- Louping ill virus
- Spanish goat encephalitis virus
- Spanish sheep encephalitis virus
- Greek goat encephalitis virus
- Turkish sheep encephalitis virus
- Powassan virus
- Omsk haemorrhagic fever virus
- Alkhurma haemorrhagic fever virus
- Kyanasur forest disease virus
- Langat virus
- Negishi Virus
- West Nile fever virus
- Yellow fever virus
- Dengue virus
- Zika virus
- Japanese Encephalitis virus
- West Nile virus
Depending on the region where serological studies are carried out, at least one of these flaviviruses may interact with the test and may lead to cross-reactive false-positive results. The detection of a TBE positive serum (either in humans or animals and by ELISA or IFA) in an area so far not known as TBE endemic can only be a first sign and has to be followed by additional tests to confirm seropositivity. The golden standard for confirmation is the neutralisation test, and even this test has some minor cross reactivity.
When sera from animals are tested as sentinel, one has to take into consideration that samples from post mortem wild animals may lead to false-positive ELISA results and some samples may be toxic to cell cultures in the neutralization assay (e.g. from horses and foxes). When planning a seroprevalence study in animals, it should also be kept in mind that some animal species may be unsuitable as sentinels due to the fact that they do not seem to seroconvert, e.g. cats.41
When animal or human sera have been found to definitely be TBE sero-positive in a given region, the next step to demonstrate that TBEV is circulating in this area is the detection of TBEV in ticks. While ticks may be found in a wide range of different landscapes and places in that region, TBE foci, that means ticks infected by TBEV, may be small, sometimes smaller than a soccer field, and the prevalence of infected ticks may be low (mostly less than 1%). Consequently, it may be very useful, to contact individuals who had TBE and/or are TBE antibody-positive and can remember where they had been bitten by a tick about one to three weeks before onset of disease. The localization of potential TBE foci can help to increase the chance to detect TBEV genome in ticks collected by flagging. This approach to identify TBE foci is much more effective than just collecting ticks in the landscape.
Investigations on TBEV or TBE in areas outside of the Eurasian continent have been successful during the last decade. TBE foci and/or TBE virus could be identified on the British Islands – now the most western part of the TBE belt – and in Japan – now the most eastern part of the TBE belt. Surprisingly, TBE virus could also be detected in Tunisia, which today is the most southern TBE virus endemic region and so far the only one on the African continent. It is assumed that migrating birds are responsible for the extension of the TBE belt by transporting ticks over wide distances. This assumption is supported by genomic sequence analyses of strains isolated from new foci and which show a close genomic relationship to strains from other regions of the TBE belt.
For the following countries preliminary data are available regarding the prevalence of TBE virus which do not yet allow a risk area assessment
Countries close to the well-known TBE belt
- Spain
The first systematic studies to investigate the probable occurrence of TBEV in Spain were carried out from 2006 to 2008. A total of more than 1800 Ixodes ricinus nymphs and 630 adult ticks collected in northern Spain were analysed by real-time reverse transcriptase PCR. All test results were negative, and the authors concluded that TBEV prevalence in northern Spain was either very low or absent in the investigated regions of northern Spain.42
A sero-epidemiological study of West Nile virus, Usutu virus and TBEV in dogs has been carried out in Spain. Flavivirus antibodies were detected in 39/815 dogs using a commercial blocking ELISA. This test system detects antibodies targeting epitopes on domain III of the envelope protein common to antigenically related flaviviruses and thus, ELISA-positive results indicate the presence of antibodies against flaviviruses. TBE positivity was confirmed using a neutralisation test in 14 dog blood samples collected in southern (Andalusia) and southwestern (Extremadura) Spain.43
A sero-epidemiological study in breeding and sport horses resident in nine autonomous communities across Spain was carried out between 2011 and 2016. A total of 14/458 (3.1%) sera were positive in a TBE serum neutralisation test.44 The authors discussed that the neutralization test used would not enable differentiation between TBEV and LIV, both members of the TBE sero-complex.
In 2011/12, a sero-epidemiological study was carried out in horses in order to assess seropositivity for various flaviviruses (Usutu virus, West Nile virus, TBEV), and 291 blood samples were taken from 172 horses.45 The IgG seroprevalence for TBE was 0.6%. Seroprevalence for WNV was 6.4% and for USV was 1.2%. The authors concluded that zoonotic arboviruses circulate in Mallorca.
- Greece
In a sero-epidemiological study across Greece, 1.7% TBE positive samples were identified in apparently healthy persons by immunofluorescence testing. It is worth mentioning that the highest seroprevalence rate was found in a region where no Ixodes ricinus ticks have been shown to be prevalent.46
In a sero-epidemiological study, 921 sera and cerebrospinal fluid from individuals with infections of the central nervous system and living in northern Greece were analysed for IgM and IgG TBE antibodies. In two percent of the general population, TBE antibodies were found (0%-5.8% in different prefectures), but TBE could not be confirmed by laboratory analyses, and the authors concluded that a flavivirus of the TBE sero-complex is circulating in the investigated region.47,48
A dog with a history of tick infection and which displayed neurological symptoms was analysed for TBE by using a commercial IgM and IgG TBE ELISA. The dog was tested positive for both IgM and IgG, and the authors concluded that diagnosis of TBE infection was confirmed by combining the clinical symptoms with this seropositivity.49 The authors stated that one limitation of the study was that no confirmation test by serum neutralisation assay was carried out.
In a review article about tick-borne pathogens and diseases in Greece,50 the authors concluded from the above cited publications that a flavivirus of the TBE sero-complex is circulating in Greece.
- Turkey
In 2007, a seroprevalence study was carried out for WNV and TBE analyzing sera from 181 samples collected at two state medical hospitals in the southeastern part of Turkey. Using a commercial TBE IgG ELSA, 10.5% were positive and 23% of the IgG positive sera were also positive in a TBE IgM ELISA. In an immunofluorescence test, 16% of the sera were positive for WNV, of which four sera were also positive for TBE. The authors concluded the possible presence of TBEV in southeastern Turkey.51
Some years later, a total of 2450 sera from healthy blood donors in central and northern Anatolia were analyzed by a commercial TBE IgG ELISA, and 47 donor samples (1.9%) were tested positive. One sample from the Black Sea region was positive in a plaque reduction neutralisation test. The authors discussed that the blood donors have had exposure to TBE virus or an antigenically similar tick-borne flavivirus.52
When 110 sera from Turkish children with fever and/or arthritis were analyzed by TBE IgM, five samples were tested positive. No sample was TBE IgG positive. Two samples were positive for WNV IgM and six sera were tested positive for WNV IgG. The authors concluded that children in Turkey were exposed to TBEV and WNV.53
In the Samsun province, a total of 419 human sera from healthy individuals were analyzed by TBE IgM and IgG ELISA. Four samples were positive for IgG and one sample tested positive for IgM. However, none of these sera were confirmed positive in a neutralization assay.54
A TBE seroprevalence study has been carried out among domestic animals in northern Turkey, and ticks were collected from animals (cattle, goat, sheep) and were analyzed for TBEV. No TBEV-specific genomic sequences were detected in a total of 2625 ticks. Screening of serum samples by a commercial TBE IgG ELISA revealed positive results in cattle (61/198, 30.8%), in goats (7/115, 6.1%) and sheep (15/147, 10.2%). The authors concluded that their study supports previous findings which indicates that TBEV is distributed in Turkey.55
- Albania and Bosnia and Hercegovina
Sero-epidemiological studies in humans and animals were carried out in the 1990s to analyse the distribution of arboviruses in Albania, and TBE positive sera were detected.56 However, the tests used in these investigations were based on indirect immunofluorescence techniques, and results may have been false-positive due to cross-reactivity with other flaviviruses. During the 2nd International Symposium on Tick-Borne Encephalitis in June 1991, Eltari reported about TBE cases in Albania.57 Later, Sherifi et al. (2018) admitted that no accurate data were available on TBE in Albania, and their attempts to detect TBEV-specific genome in ticks collected by flagging had been negative.58
There is only one report from Bosnia mentioning human TBE cases (Burger, 2017).
However, Zlobin et al. (2006) isolated three Siberian TBEV subtype strains – Bosnian lineage, two strains from one male and one female Dermacentor marginatus and 1 strain isolated from Ixodes ricinus nymph. 59,60
In total, the southwestern Balkans (Albania, Bosnia and Herzegovina, Macedonia, Montenegro) have only a few or no studies about TBE and TBE related reports.
- North Macedonia
In a study to assess the prevalence of antibodies to Borrelia burgdorferi and TBEV in North Macedonia and Serbia, one serum sample from a female in North Macedonia was positive for neutralising TBE antibodies. This result suggests the potential existence of TBE foci in North Macedonia, however, there is still the alternative explanation that this person was exposed to TBEV during a short stay in Austria.61
- Afghanistan
In a serological study dealing with the seroprevalence of various flaviviruses, a commercial IgG and IgM ELISA was used to assess seropositivity among individuals in Afghanistan. A total of 30.8% of the sera were IgG-positive for TBE, and 20.6% were co-reactive in a WNV-ELISA. 2.2% of the sera were TBE-IgM positive. The authors concluded that TBEV may circulate in Afghanistan.62 However, these high prevalence rates may be due to another circulating flavivirus of the tick-borne mammalian group, Royal Farm virus, which was isolated in Afghanistan in 1968 from soft ticks. With no NT testing available the situation remains unclear.
- Georgia
In Georgia, 7% of acute febrile patients showed TBEV seropositivity.63 Non-published data show that TBEV-EU may circulate in Georgia. The interpretation of the data is unclear.
- Iran
Raw milk samples collected from local dairy markets around Qazvin, a city in northern Iran, have been analysed by using nested and multiplex PCR methods for the presence for various foodborne and zoonotic viruses. TBEV genomic sequences were detected in 42/492 (18.91%) of the analysed samples.64 The authors concluded that the presence of TBEV in raw milk may pose an immediate health risk for milk and dairy consumers, even without any reported TBE cases in the Qazvin area.
In a conference report,65 data on the presence of TBEV in raw milk samples from sheep (4.4%), goat (4.4%) and cows (0%) in northwest Iran were presented, and TBE antibodies evaluated by ELISA were found in the milk of sheep (4.4%), goats (2.2%) and cows (1.1%). However, we did not find these data anywhere in a peer-reviewed journal.
A cross-sectional sero-epidemiological study has been carried out in rural areas in northern Iran in order to analyse the prevalence of TBE antibodies among the general population using a commercial TBEV ELISA IgG kit. A total of 16/448 serum samples tested positive. The authors discuss that there are uncertainties about the accuracy of positive results on serological tests, such a ELISA, owing to the antigenic cross-reactivity among flaviviruses, and they concluded that confirmation is needed by neutralisation test and that the results should be interpreted with caution.66
- Central Asian countries
Within the Central Asian countries there are reports of TBE in Kazakhstan and Kirgizstan (see country chapters), the only other single report without any further details is from Turkmenistan.67
Countries remote from the TBE belt
- Comores
A cross-sectional survey of arboviral infections in humans was conducted on three islands of the Union of Comores in 2011. Using a commercial TBE IgG ELISA, 3/400 sera were positive in the TBE ELISA, but no neutralisation/confirmation tests were carried out.68
- Kenya
A seroprevalence study was carried out in Kenya in 2000 to 2004 to evaluate the prevalence of arboviral infections. A high seroprevalence of TBE IgG (16% in older persons, 6% in children) was found using a commercial indirect immunofluorescent test, and the authors concluded that this was a result of cross-reactions amongst related flaviviruses.69
- Djibouti
In a sero-epidemiological study carried out in Djibouti to assess the burden of a variety of arboviral diseases, antibodies to Dengue were the most frequent (21.8).In 2/1045 sera, TBE antibodies were detected using a commercial ELISA. While the first serum sample was negative in a TBE specific neutralisation assay and negative also for Alkhurma virus, the second serum was slightly positive for both viruses. The authors discussed that these two TBE seropositive individuals may have been migrants with a specific exposure to tick bites in a rural environment.70
- Vietnam
TBE sero-epidemiological analyses using an indirect immunofluorescence antibody test (IFAT) of sera from humans and rats gave positivity rates of 47.3% and 5.4%, respectively. The authors concluded that the TBE reactivity in both humans and rodents detected by the IFAT most likely reflected cross-reactivity with other flaviviruses, especially with Dengue virus and Japanese encephalitis virus.71
- Malaysia
Among farms workers, a TBE seropositivity of 36.5% was found using a commercial TBE IgG ELISA. However, when testing these sera against three antigenically related flaviviruses (DEN, WN, JEV), only 4.2% of the sera did not show cross-reactivity. The authors discussed that the remaining TBE seropositivity may be due to cross-reactivity to Langat virus and they concluded that even a virus neutralisation test could still lead to false TBE seropositivity results.72
Summary
In this book, we did all possible to identify predisposed, imperiled, affected and endemic areas. For the countries mentioned in the end, surveillance data using TBEV-NT would be most simple to confirm TBEV circulation – which would be relevant for travelers. In endemic countries reporting should be enhanced and commercial tests for TBE should be easily accessible. Clearly, the country-specific information on TBE – epidemiology is still scarce and results in relevant underdiagnosis.
The following country reports in Chapter 13 provide standardized information, as available on:
- The risk area assessment based on the ECDC definition (see above)
- The history of TBE in the respective country as well as various specific aspects
- Virus, vector, transmission of TBE
- TBE-reporting and prevention by vaccination
- TBE case numbers over time
- Local demographics of TBE
- TBEV-isolation and TBE cases – risk area distribution
The risk map in Chapter 13 shows the extent of TBEV based on documented TBE cases, TBEV infection, as well as on the detection of TBEV-circulation in nature (i.e., imperiled, affected and endemic areas). The map does not reflect the incidence of the disease or the universal prevalence of the virus in a given area. As the quality, intensity and complete-ness of epidemiological surveillance varies between different countries, the map presented here must be incomplete, and very likely TBEV infections and thus TBE may occur in additional (“new”) areas.
The risk map distribution is based on the second and third level of the Nomenclature of territorial units for statistics (NUTS) used for subnational analysis, depending on availability (Eurostat, the statistical office of the European Union. Nomenclature of Territorial Units for Statistics. Luxembourg: Eurostat. [Accessed: 7 Mar 2023]. Available from: http://ec.europa.eu/eurostat/web/nuts/overview)
Acknowledgment
We thank all authors and the co-editors of THE TBE BOOK, who left no stone unturned in their efforts to find current country-specific information on as many countries as possible and we would like to thank again all authors of Chapter 13 for providing their timely reports.
Contact
Wilhelm Erber
Wilhelm.Erber@gmail.com
Authors
Wilhelm Erber, Michael Broeker, Gerhard Dobler, Lidia Chitimia-Dobler, Heinz-Josef Schmitt
Citation
Erber W, Broeker M, Dobler G, Chitimia-Dobler L, Schmitt HJ. Epidemiology of TBE. Chapter 12. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ, eds. The TBE Book. 7th ed. Singapore: Global Health Press; 2024. doi:10.33442/26613980_12-7
References
- Mazanik A. Arbovirology and Cold War Collaborations: A Transnational History of the Tick-borne Encephalitis Vaccine, 1930-1980. J Hist Med Allied Sci. Published online September 8, 2023. doi:10.1093/jhmas/jrad054
- Kollaritsch H, Krasilnikov V, Holzmann H, et al. Background document on vaccines and vaccination against tick–borne encephalitis. Geneva, WHO Strategic Advisory Group of Experts on Immunization. Accessed 29 May, 2012. https://www.who.int/immunization/sage/6_TBE_backgr_18_Mar_net_apr_2011.pdf
- Tick-borne encephalitis and haemorrhagic fever with renal syndrome in Europe. Report on a WHO meeting. EURO Rep Stud. 1986;(104):1-79.
- Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86(24):241-256.
- World Health Organization. World Health Organization model list of essential medicines: 21st list 2019, W.H.O. Geneva, Editor. Accessed 27 April, 2024. https://www.who.int/medicines/publications/essentialmedicines/en/.
- European Commission. Commission Decision 2002/253/EC of 19 March 2002 laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. EC. 2012.
- Donoso Mantke O, Escadafal C, Niedrig M, Pfeffer M, Working Group For Tick-Borne Encephalitis Virus C. Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill. 2011;16(39):19976. Published 2011 Sep 29. doi:10.2807/ese.16.39.19976-en
- Stefanoff P, Zielicka-Hardy A, Hlebowicz M, et al. New endemic foci of tick-borne encephalitis (TBE) identified in districts where testing for TBE was not available before 2009 in Poland. Parasit Vectors. 2013;6:180. Published 2013 Jun 18. doi:10.1186/1756-3305-6-180
- Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19(1):69-76. doi:10.3201/eid1901.120458
- Bogovič P, Kastrin A, Lotrič-Furlan S, et al. Clinical and Laboratory Characteristics and Outcome of Illness Caused by Tick-Borne Encephalitis Virus without Central Nervous System Involvement. Emerg Infect Dis. 2022;28(2):291-301. doi:10.3201/eid2802.211661
- Kjær LJ, Johansson M, Lindgren PE, et al. Potential drivers of human tick-borne encephalitis in the Örebro region of Sweden, 2010-2021. Sci Rep. 2023;13(1):7685. Published 2023 May 11. doi:10.1038/s41598-023-34675-x
- Albinsson B, Hoffman T, Kolstad L, et al. Seroprevalence of tick-borne encephalitis virus and vaccination coverage of tick-borne encephalitis, Sweden, 2018 to 2019. Euro Surveill. 2024;29(2):2300221. doi:10.2807/1560-7917.ES.2024.29.2.2300221
- Weststrate AC, Knapen D, Laverman GD, et al. Increasing evidence of tick-borne encephalitis (TBE) virus transmission, the Netherlands, June 2016. Euro Surveill. 2017;22(11):30482. doi:10.2807/1560-7917.ES.2017.22.11.30482
- Dekker M, Laverman GD, de Vries A, Reimerink J, Geeraedts F. Emergence of tick-borne encephalitis (TBE) in the Netherlands. Ticks Tick Borne Dis. 2019;10(1):176-179. doi:10.1016/j.ttbdis.2018.10.008
- Superior Health Council. Vaccination against Tick-Borne Encephalitis. 2019;9435
- Holding M, Dowall SD, Medlock JM, et al. Detection of new endemic focus of tick-borne encephalitis virus (TBEV), Hampshire/Dorset border, England, September 2019. Euro Surveill. 2019;24(47):1900658. doi:10.2807/1560-7917.ES.2019.24.47.1900658
- Holding M, Dowall SD, Medlock JM, et al. Tick-Borne Encephalitis Virus, United Kingdom. Emerg Infect Dis. 2020;26(1):90-96. doi:10.3201/eid2601.191085
- Daniel M, Danielová V, Kríz B, Jirsa A, Nozicka J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in central Europe. Eur J Clin Microbiol Infect Dis. 2003;22(5):327-328. doi:10.1007/s10096-003-0918-2
- Holzmann H, Aberle SW, Stiasny K, et al. Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Emerg Infect Dis. 2009;15(10):1671-1673. doi:10.3201/eid1510.090743
- Lukan M, Bullova E, Petko B. Climate warming and tick-borne encephalitis, Slovakia. Emerg Infect Dis. 2010;16(3):524-526. doi:10.3201/eid1603.081364
- Briggs BJ, Atkinson B, Czechowski DM, et al. Tick-borne encephalitis virus, Kyrgyzstan. Emerg Infect Dis. 2011;17(5):876-879. doi:10.3201/eid1705.101183
- Heinz FX, Stiasny K, Holzmann H, et al. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Euro Surveill. 2015;20(13):9-16. Published 2015 Apr 2. doi:10.2807/1560-7917.es2015.20.13.21077
- Soleng A, Edgar KS, Paulsen KM, et al. Distribution of Ixodes ricinus ticks and prevalence of tick-borne encephalitis virus among questing ticks in the Arctic Circle region of northern Norway. Ticks Tick Borne Dis. 2018;9(1):97-103. doi:10.1016/j.ttbdis.2017.10.002
- Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip Perspect Infect Dis. 2009;2009:593232. doi:10.1155/2009/593232
- WHO. World health report of an international consultation: methodology for risk mapping of the international spread of vector-borne diseases via air travel. 2018: Geneva, Switzerland.
- Borde JP, Glaser R, Braun K, et al. Decoding the Geography of Natural TBEV Microfoci in Germany: A Geostatistical Approach Based on Land-Use Patterns and Climatological Conditions. Int J Environ Res Public Health. 2022;19(18):11830. Published 2022 Sep 19. doi:10.3390/ijerph191811830
- Suess J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2011;2(1):2-15. doi:10.1016/j.ttbdis.2010.10.007
- Domanovic D, Giesecke J. How to define an area where transmission of arthropod-borne disease is occurring? Euro Surveill. 2012;17(20).
- Dai X, Shang G, Lu S, Yang J, Xu J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg Microbes Infect. 2018;7(1):74. doi:10.1038/s41426-018-0081-6
- Jääskeläinen A, Tonteri E, Pieninkeroinen I, et al. Siberian subtype tick-borne encephalitis virus in Ixodes ricinus in a newly emerged focus, Finland. Ticks Tick Borne Dis. 2016;7(1):216-223. doi:10.1016/j.ttbdis.2015.10.013
- Jääskeläinen AE, Tonteri E, Sironen T, Pakarinen L, Vaheri A, Vapalahti O. European subtype tick-borne encephalitis virus in Ixodes persulcatus ticks. Emerg Infect Dis. 2011;17(2):323-325. doi:10.3201/eid1702.101487
- Lundkvist k, Vene S, Golovljova I, et al. Characterization of tick-borne encephalitis virus from Latvia: evidence for co-circulation of three distinct subtypes. J Med Virol. 2001;65(4):730-735. doi:10.1002/jmv.2097
- Golovljova I, Vene S, Sjölander KB, Vasilenko V, Plyusnin A, Lundkvist A. Characterization of tick-borne encephalitis virus from Estonia. J Med Virol. 2004;74(4):580-588. doi:10.1002/jmv.20224
- Yurchenko OO, Dubina DO, Vynograd NO, Gonzalez JP. Partial Characterization of Tick-Borne Encephalitis Virus Isolates from Ticks of Southern Ukraine. Vector Borne Zoonotic Dis. 2017;17(8):550-557. doi:10.1089/vbz.2016.2094
- Tkachev SE, Babkin IV, Chicherina GS, et al. Genetic diversity and geographical distribution of the Siberian subtype of the tick-borne encephalitis virus. Ticks Tick Borne Dis. 2020;11(2):101327. doi:10.1016/j.ttbdis.2019.101327
- Demina TV, Dzhioev YP, Verkhozina MM, et al. Genotyping and characterization of the geographical distribution of tick-borne encephalitis virus variants with a set of molecular probes. J Med Virol. 2010;82(6):965-976. doi:10.1002/jmv.21765
- Adelshin RV, Melnikova OV, Karan LS, Andaev EI, Balakhonov SV. Complete Genome Sequences of Four European Subtype Strains of Tick-Borne Encephalitis Virus from Eastern Siberia, Russia. Genome Announc. 2015;3(3):e00609-15. Published 2015 Jun 18. doi:10.1128/genomeA.00609-15
- Evstaf’ev IL. Itogi 20-letnego izucheniia kleshchevogo éntsefalita v Krymu [Results of the 20-year study of tick-borne encephalitis in Crimea]. Zh Mikrobiol Epidemiol Immunobiol. 2001;(2):111-114.
- Ponomareva EP, Mikryukova TP, Gori AV, et al. Detection of Far-Eastern subtype of tick-borne encephalitis viral RNA in ticks collected in the Republic of Moldova. J Vector Borne Dis. 2015;52(4):334-336.
- Topp AK, Springer A, Mischke R, et al. Seroprevalence of tick-borne encephalitis virus in wild and domestic animals in northern Germany. Ticks Tick Borne Dis. 2023;14(6):102220. doi:10.1016/j.ttbdis.2023.102220
- Barandika JF, Hurtado A, Juste RA, García-Pérez AL. Seasonal dynamics of Ixodes ricinus in a 3-year period in northern Spain: first survey on the presence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2010;10(10):1027-1035. doi:10.1089/vbz.2009.0148
- García-Bocanegra I, Jurado-Tarifa E, Cano-Terriza D, Martínez R, Pérez-Marín JE, Lecollinet S. Exposure to West Nile virus and tick-borne encephalitis virus in dogs in Spain. Transbound Emerg Dis. 2018;65(3):765-772. doi:10.1111/tbed.12801
- Camino E, Schmid S, Weber F, et al. Detection of antibodies against tick-borne encephalitis flaviviruses in breeding and sport horses from Spain. Ticks Tick Borne Dis. 2020;11(5):101487. doi:10.1016/j.ttbdis.2020.101487
- Vanhomwegen J, Beck C, Desprès P, et al. Circulation of Zoonotic Arboviruses in Equine Populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. 2017;17(5):340-346. doi:10.1089/vbz.2016.2042
- Antoniadis A, Alexiou-Daniel S, Malissiovas N, et al. Seroepidemiological survey for antibodies to arboviruses in Greece. In: Calisher CH, ed. Hemorrhagic Fever with Renal Syndrome, Tick- and Mosquito-Borne Viruses. Springer Vienna; 1990:277-285. doi:10.1007/978-3-7091-9091-3_31
- Pavlidou V, Geroy S, Diza E, Antoniadis A, Papa A. Epidemiological study of tick-borne encephalitis virus in northern Greece. Vector Borne Zoonotic Dis. 2007;7(4):611-615. doi:10.1089/vbz.2007.0107
- Pavlidou V, Gerou S, Diza E, Antoniadis A, Papa A. Genetic study of the distribution of Greek goat encephalitis virus in Greece. Vector Borne Zoonotic Dis. 2008;8(3):351-354. doi:10.1089/vbz.2007.0215
- Sioutas G, Tsakou K, Top C, Jongejan F, Papadopoulos E. First clinical case of tick-borne encephalitis (TBE) in a dog in Greece. Ticks Tick Borne Dis. 2023;14(6):102226. doi:10.1016/j.ttbdis.2023.102226
- Efstratiou A, Karanis G, Karanis P. Tick-Borne Pathogens and Diseases in Greece. Microorganisms. 2021;9(8):1732. Published 2021 Aug 14. doi:10.3390/microorganisms9081732
- Ergunay K, Ozer N, Us D, et al. Seroprevalence of West Nile virus and tick-borne encephalitis virus in southeastern Turkey: first evidence for tick-borne encephalitis virus infections. Vector Borne Zoonotic Dis. 2007;7(2):157-161. doi:10.1089/vbz.2006.0574
- Ergünay K, Saygan MB, Aydoğan S, et al. Confirmed exposure to tick-borne encephalitis virus and probable human cases of tick-borne encephalitis in Central/Northern Anatolia, Turkey. Zoonoses Public Health. 2011;58(3):220-227. doi:10.1111/j.1863-2378.2010.01342.x
- Yilmaz H, Barut K, Karakullukcu A, et al. Serological Evidence of Tick-Borne Encephalitis and West Nile Virus Infections Among Children with Arthritis in Turkey. Vector Borne Zoonotic Dis. 2019;19(6):446-449. doi:10.1089/vbz.2018.2349
- Aslan Başbulut E, Gözalan A, Sönmez C, et al. Samsun Kırsalında Borrelia burgdorferi ve Kene Ensefaliti Virusu Seroprevalansının Araştırılması [Seroprevalence of Borrelia burgdorferi and tick-borne encephalitis virus in a rural area of Samsun, Turkey]. Mikrobiyol Bul. 2012;46(2):247-256.
- Asal G, Tamer C, Albayrak H. Serological survey and molecular investigation of tick-borne encephalitis virus in Northern Turkey. Etlik Veteriner Mikrobiyoloji Dergisi. 2022;33(1):34-39. doi:10.35864/evmd.1064554
- Eltari E. Epidemiology of tick-borne encephalitis in Albania. Ellipse. 1991;29:449–450.
- Eltari E, et al. Some data on arboviruses, especially tick-borne encephalitis, in Albania. Giornale di Malattie Infettive e Parassitarie. 1993;45:404.
- Sherifi K, Rexhepi A, Berxholi K, et al. Crimean-Congo Hemorrhagic Fever Virus and Borrelia burgdorferi sensu lato in Ticks from Kosovo and Albania. Front Vet Sci. 2018;5:38. Published 2018 Mar 6. doi:10.3389/fvets.2018.00038
- Zlobin VI, Verkhozina MM, Demina TV, et al. Vopr Virusol. 2007;52(6):4-13.
- Omeragic J. TICKS AND TICK-BORNE PATHOGENS IN BOSNIA AND ERZEGOVINA, in Specialty training course for young scientists of West Balkan countries “New methods in Parasitology: Tick-borne disease research and targeted selective anthelmintic treatment”. 2013: Munich, Germany.
- Jakimovski D, Mateska S, Dimitrova E, et al. Tick-Borne Encephalitis Virus and Borrelia burgdorferi Seroprevalence in Balkan Tick-Infested Individuals: A Two-Centre Study. Pathogens. 2023;12(7):922. Published 2023 Jul 9. doi:10.3390/pathogens12070922
- Elyan DS, Moustafa L, Noormal B, et al. Serological evidence of flaviviruses infection among acute febrile illness patients in Afghanistan. J Infect Dev Ctries. 2014;8(9):1176-1180. Published 2014 Sep 12. doi:10.3855/jidc.4183
- Kuchuloria T, Imnadze P, Mamuchishvili N, et al. Hospital-Based Surveillance for Infectious Etiologies Among Patients with Acute Febrile Illness in Georgia, 2008-2011. Am J Trop Med Hyg. 2016;94(1):236-242. doi:10.4269/ajtmh.15-0400
- Pakbin B, Rossen JWA, Brück WM, et al. Prevalence of foodborne and zoonotic viral pathogens in raw cow milk samples. FEMS Microbiol Lett. 2022;369(1):fnac108. doi:10.1093/femsle/fnac108
- Parsadanians A, Mirshahabi H, Yavarmanesh M. Frequency of Tick-Borne Encephalitis Virus (TBEV) in Raw Milk Samples in Zanjan, Northwest of Iran. 2016.
- Salehi-Vaziri M, Pouriayevali MH, Azad-Manjiri S, Vasmehjani AA, Baniasadi V, Fazlalipour M. The seroprevalence of tick-borne encephalitis in rural population of mazandaran province, northern iran(2018 – 2019). Arch Clin Infect Dis. 2020;15(1). doi:10.5812/archcid.98867
- Atkinson B, Hewson R. Emerging arboviruses of clinical importance in Central Asia. J Gen Virol. 2018;99(9):1172-1184. doi:10.1099/jgv.0.001125
- Dellagi K, Salez N, Maquart M, et al. Serological Evidence of Contrasted Exposure to Arboviral Infections between Islands of the Union of Comoros (Indian Ocean). PLoS Negl Trop Dis. 2016;10(12):e0004840. Published 2016 Dec 15. doi:10.1371/journal.pntd.0004840
- Sutherland LJ, Cash AA, Huang YJ, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg. 2011;85(1):158-161. doi:10.4269/ajtmh.2011.10-0203
- Andayi F, Charrel RN, Kieffer A, et al. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PLoS Negl Trop Dis. 2014;8(12):e3299. Published 2014 Dec 11. doi:10.1371/journal.pntd.0003299
- Van Cuong N, Carrique-Mas J, Vo Be H, et al. Rodents and risk in the Mekong Delta of Vietnam: seroprevalence of selected zoonotic viruses in rodents and humans. Vector Borne Zoonotic Dis. 2015;15(1):65-72. doi:10.1089/vbz.2014.1603
- Mohd Shukri M, Ling Kho K, Ghane Kisomi M, et al. Seroprevalence report on tick-borne encephalitis virus and Crimean-Congo hemorrhagic fever virus among Malaysian’s farm workers. BMC Public Health. 2015;15:704. Published 2015 Jul 24. doi:10.1186/s12889-015-1901-4