Phung Lang, Rahel Ackermann-Gäumann
Key points
- The identification of TBE endemic areas is crucial to inform national and international TBE risk management programs. However, identification of TBE endemic areas remains incomplete.
- The risk of tick-borne disease is predicted to increase with climate change through several mechanisms, but the relationship between climate and tick-borne disease is complex and influenced by both environmental and human factors.
- Uptake and compliance with TBE vaccination in Europe vary greatly, with overall low rates.
- Disparities in TBE awareness and vaccine uptake exist between endemic and non-endemic countries. Targeted education, involvement of healthcare professionals, and accessible vaccination strategies are needed to address barriers and improve prevention for those living in or travelling to TBE endemic areas.
- Because children also suffer from long-term cognitive impairment and because TBE cases in children are likely to be underreported, TBE vaccination is important for this age group.
- Increasing vaccination rates across all age groups is the most effective and efficient strategy to reduce the burden of TBE and protect the overall population’s health.
- To effectively manage and prevent the spread of TBE, a comprehensive One Health approach must consider the complex interactions between humans, animals, ticks, and the environment.
Introduction
Public health measures are a key strategy for reducing the transmission of pathogens with epidemic potential. These measures encompass vaccination programs and non-pharmaceutical interventions that can be implemented by individuals, institutions, communities, local and national governments, and international bodies to slow or stop the spread of an infectious disease. TBE requires significant public health attention due to its potential to harm individuals residing in or traveling to TBE endemic areas. The disease can lead to long-term disability and even death. It is important to inform the public about the risks associated with TBE and provide an appropriate public health response.
Reporting and surveillance
TBEV is found in natural foci, which are areas where the virus circulates among ticks and reservoir hosts. As a result, TBE is limited to specific geographical regions, resulting in TBE endemic areas.1–3 More than 25 countries in Northern, Central, and Eastern Europe have one or more areas where TBE is endemic,4 with the highest reporting rates in the Baltic States, Slovenia, and the Czech Republic.5 Together with Russia and part of eastern Asia, these countries form what is known as the “TBE belt”.6 The incidence of TBE has increased over the past 25 years,7,8 with a northwestward spread in continental Europe, including to regions and altitudes previously believed to be free of the virus.1,9–11 The number of reported TBE cases in Europe in 2020 was twice that of 2015;9 nearly 30,000 cases were reported in the EU/EEA countries between 2012 and 2020.11 However, annual case reporting fluctuates widely due to various factors.1
Since 2012, the European Centre for Disease Prevention and Control (ECDC) has required all European Union member states, as well as Iceland and Norway, to report their TBE data annually to the European Surveillance System (TESSy).12 In 2022, 43% of European countries13 used the latest diagnostic criteria introduced by the ECDC in 2018.14 In some countries that do not use the ECDC criteria, such as Italy, national diagnostic criteria are largely similar to the ECDC criteria. Therefore, reported TBE case numbers may not differ significantly.1,14 However, this may not be the case in countries that have key differences in requirements for the confirmation of a TBE case, such as in Germany, where clinical signs may be limited to non-specific symptoms (i.e., without CNS symptoms).1,15–17 Country data on TBE prevalence is difficult to compare due to differences in case definitions between countries, resulting in varying degrees of accuracy.1,9
TBE is typically an acute disease, and progression may terminate after the first phase, which is called the “abortive” clinical pattern. This form of TBE may be asymptomatic or manifest as a mild febrile illness, including symptoms such as headache, fever, fatigue, myalgia, anorexia, nausea, and vomiting, without progression to any form of encephalitis.18,19 However, only a few countries, namely Austria, Latvia, Germany, and Slovenia, collect data on nonspecific non-CNS symptoms.1,15,20–23 Additionally, mild CNS symptoms may go unreported since they do not fulfill the ECDC criteria, leading to underreporting of TBE. This is particularly noteworthy in pediatric patients, where symptoms are often mild and can be misdiagnosed.1 are very likely to be underreported compared to adults, as up to two-thirds of pediatric TBE cases are missed.9,24,25
Clinicians who do not test for TBEV infection due to a lack of recognition of the possibility of CNS inflammation may impact the number of reported TBE cases. Furthermore, if they suspect CNS inflammation, they may be less inclined to perform a CSF examination that supports a TBE diagnosis.1
Access to diagnostic tests for TBE is limited, as is knowledge on their appropriate use.1 Serological assays are the preferred method for TBE diagnosis.26 However, interpreting serologic test results is challenging due to the high cross-reactivity of the antigenic structure among orthoflaviviruses, particularly in areas where other orthoflaviviruses co-circulate or where vaccination against other orthoflaviviruses is common.27 Improved laboratory capacities and implementation of neutralization assays in these countries could improve identification of TBE by distinguishing it from other orthoflaviviral infections.1,28 Due to strict biosafety regulations in a number of Western countries, the performance of neutralization assays is restricted to laboratories equipped with a biosafety level 3 facility (biosafety level 4 in the United States). Therefore, alternative assays not requiring the work with infectious viruses could also be of value.29,30
Accurately determining the tick populations infected with TBEV and the number of human TBE cases is crucial for defining TBE risk areas. Endemic areas, which are risk areas where recurrent transmission of TBEV to humans occurs over several seasonal cycles,31 must be documented in most countries to make targeted vaccination recommendations.1,32,33
The geographic restriction of TBE allows for targeted surveillance in high-risk areas. However, incomplete surveillance can lead to a poor understanding of TBE endemic areas and potentially inadequate vaccine recommendations.1 This was demonstrated in Poland, where numerous new endemic districts were identified, including foci far away from previously known endemic districts, during an enhanced surveillance project.34 Restricted surveillance may hinder the early identification of new TBE endemic areas, thereby increasing the risk of TBEV infection for the public. Moreover, designating areas as endemic or high-risk may limit awareness and diagnosis of TBE in non-endemic areas, despite a national obligation to report TBE cases. This may lead to a decrease in the ability to detect cases of TBE in areas where the disease was not previously present, as well as in the diagnosis of imported cases of TBE.1
Overall, the identification of TBE endemic areas is crucial to inform national and international TBE risk management programs.1 However, identification of TBE endemic areas remains incomplete, and TBE surveillance in Europe is generally sporadic rather than systematic.9 TBE cases are likely to be underreported, and the true burden of TBE disease is significantly underestimated.9
Impact of climate change on tick-borne encephalitis
Infection transmission occurs when the activities of reservoirs, vectors, and humans overlap, with variations depending on the pathogen and location. Climate change has the potential to affect all of these stages and their interactions.35 Climate change is expected to increase the risk of ticks and tick-borne diseases in a number of ways.36–38 However, the relationship between tick-borne diseases and climate is not linear. Rather, it is influenced by other environmental and human factors.36–41
Ixodes ricinus, the primary vector of TBEV in Europe, is particularly sensitive to environmental conditions, as this tick species requires a microclimatic relative humidity of at least 80% during its extended non-parasitic periods to avoid lethal dehydration. While changes in climate and the duration of different seasons will affect tick survival, activity, and development, there is insufficient evidence to support the concept that an increase in temperatures will directly lead to a higher tick abundance simply by accelerating developmental rates. Instead, shifts in development rates will alter patterns of seasonal activity.35,42
Indirect effects of climate change will affect the number of infected ticks by affecting vegetation.35 For example, there is a link between tree mast, rodent population dynamics, nymphal tick density, and the incidence of human TBE two years later.43–46 While climate warming has increased seed production in certain trees, mast seeding events have decreased.47 A warming climate in central Europe is expected to lead to shifts in dominant tree species, resulting in a favorable microclimate for the survival of the free-living tick stages.35
Climate change will indirectly affect the transmission of tick-borne pathogens by affecting the survival and abundance of tick maintenance hosts, such as deer, and pathogen reservoir hosts, such as rodents and birds.35,48,49 Increasing temperatures will expand the distribution range of both reservoir and tick maintenance hosts50,51 as well as their abundance and activity.51,52
Climate change may affect disease risk by influencing long-term land use (e.g., farming, tourism).35 Human behavior is also expected to adapt as the climate changes. People may resume outdoor activities earlier in the spring and maintain them longer in the fall, thereby increasing the duration of annual tick contact for both animal hosts and humans. The risk of climate change to human exposure is more likely to be associated with shorter winters than with extreme summer heat.36–38
The influence of climatic factors on virus replication has not been elucidated. However, there is evidence that certain TBEV strains can adapt to different environmental temperatures within the tick.53 The spread of TBEV infection locations is significantly more frequent where precipitation and temperature are high in summer and frost days are low in winter.54 With projected climate change, the range of I. ricinus can expand to higher latitudes, particularly in northern and eastern Europe, and to higher altitudes.10,55–58
While I. ricinus is the primary vector of TBEV, the virus has also been isolated from other tick species. Therefore, changes in the range of these species may also affect the risk of contracting TBE. Statistical habitat models predict a further distribution and a potential long-term establishment of the tick species Dermacentor reticulatus and Hyalomma marginatum.35,59
Taken together, climate change can affect the transmission of tick-borne diseases by influencing the survival, abundance, and activity of ticks, as well as their hosts. The relationship between tick-borne diseases and climate is complex. Changes in temperature, precipitation, and vegetation are expected to shift the geographical distribution and incidence of diseases like TBE. This is due to factors such as changes in tick activity patterns and the expansion of tick habitats, which increase the risk of TBE in certain regions.
TBE and tick awareness and risk subjects and general protective measures
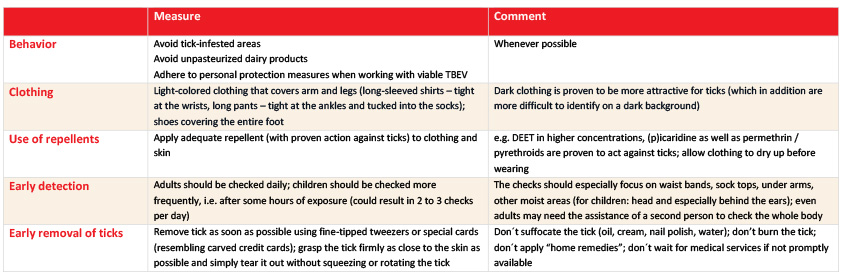
As there is currently no specific treatment available for TBE infection, prevention is strongly recommended. Vaccination is the most effective mechanism of protection against the development of TBE, in addition to the elimination of all possible exposures. General protective measures and behaviors are recommended as primary and secondary preventive measures, as summarized in Table 1.60 The best way to reduce the risk of exposure is to avoid tick-infested areas, especially during the peak tick season in spring and late summer. However, it is not always possible to avoid exposure to ticks, especially for residents of endemic areas. Therefore, it is recommended to wear protective clothing with long sleeves and long trousers tucked into socks or boots, to use repellents on exposed skin, and to impregnate clothing with an acaricide (such as permethrin or pyrethroids). After a tick bite, TBEV is immediately transmitted to the host through the tick’s saliva. It is recommended to remove the tick as soon as possible, even if it is already firmly attached to the skin, to prevent other potential infections. In the event of a tick bite, the tick should be removed using fine-tipped tweezers/forceps or a specially designed tick card/removal tool by pulling straight out without squeezing or twisting the tick. Unpasteurized dairy products in tick-infested areas may also contain TBE;61–63 avoid eating or drinking unpasteurized milk and cheese from goats, sheep or cows from these areas.
The main individual-level risk factors for TBE can be divided into two categories: behavioral and occupational risks, and biological risks. Behavioral and occupational risks include factors that increase the likelihood of exposure to ticks and contracting TBE. Forestry workers, farmers and hunters are at higher risk of contracting TBE, due to the nature of their work. Additionally, leisure activities in the countryside also increase the risk of exposure to TBE, which are more common among older individuals with more leisure time. Studies of clinical TBE cases in Switzerland found that around 80-90% of patients with TBE or Lyme borreliosis contracted the disease during leisure activities.64–66 Another related risk is the geographic region in which an individual lives, works, or spends leisure time.64–68
Biological risks for TBE disease include gender and age.12,65,66,69,70 Cases are more common in men, but this may be due to an increased risk of exposure rather than a different immune response to TBE in men and women. Both the incidence and severity of the disease increase with age.70,71 Existing comorbidities, immunosuppression and certain genetic predispositions also increase the risk of severe disease following exposure but not of the risk of exposure itself. Adults over the age of 50 not only have an increased incidence of TBE, but they also tend to experience more severe disease and have a higher risk of lasting neurological sequelae.70–72 Immunocompromised individuals, such as immunosuppressed patients, organ or hematopoietic stem cell transplant recipients, and HIV-infected individuals, are particularly susceptible to TBE and often experience severe or fatal disease.70,73–76
Published research has identified several factors associated with awareness of TBE and uptake of TBE vaccines. A recent study assessed TBE awareness and vaccination rates in 2020 in 20 European countries.67 Of these, 14 countries were identified as TBE endemic and 6 as non-endemic. The results showed that there was a difference in TBE awareness (74% vs. 30%) and TBE vaccine awareness (56% vs. 12%) between endemic and non-endemic countries.67 Motivating predictors of TBE vaccination include recommendation from a physician (in both endemic and non-endemic countries), personal or occupational risk exposure, fear of TBE, dog ownership, experience with tick-related health problems, desire to avoid contracting the disease, trust in vaccine recommendations, frequent outdoor activities, gardening and travel to an endemic area.67,68,77–80 While those who were vaccinated against TBE were better informed about TBE disease than non-vaccinated individuals in a non-endemic TBE area, getting a TBE vaccination was not associated with a reduced uptake of general protective measures.81 Barriers to TBE vaccination include not living in or visiting risk areas, low risk perception, fear of adverse events following vaccination, lack of information about TBE and the vaccine, unavailability of the TBE vaccine, and the belief that vaccination is unnecessary.67,68,78,79
Individual-level risk factors for TBE include higher exposure risks for forestry workers and individuals engaging in outdoor activities in endemic areas. Additionally, age, gender and comorbidities can contribute to the degree of susceptibility to TBE. The recognition of differences in TBE awareness and vaccine uptake between endemic and non-endemic countries underlines the need for targeted education, involvement of health professionals, and accessible vaccination strategies to eliminate barriers and enhance prevention.
Vaccination schedules and recommendations
There are six licensed vaccines available, all of which use inactivated whole virus strains. These vaccines can be grouped into European, Russian, and Chinese vaccines.82 Currently, two European vaccines are available in many European countries and Canada, and one is available in the United States. They are based on the Austrian isolate Neudoerfl (FSME-IMMUN) and the German isolate K23 (Encepur), both TBEV-Eu strains. Additionally, licensed vaccines in Russia and some neighboring countries are based on the Russian TBEV-FE isolate Sofjin (TBE vaccine Moscow and Tick-E-Vac/Klesch-E-Vac) and TBEV-FE strain 205 (EnceVir). In China, SenTaiBao, which is based on the Chinese TBEV-FE strain Sen-Zhang, has been approved as a TBEV vaccine (reviewed in17,19,82–85). Pediatric formulations are available for FSME-IMMUN, Encepur, TBE vaccine Moscow, Tick-E-Vac, and EnceVir vaccines.19 The standard immunization schedule for all vaccines, except for Sen Tai Bao which has only two doses, consists of three doses. The initial vaccination is followed by a second injection 4-12 weeks later, and a third injection is given 5-12 months later, with variations in the specific intervals between vaccine brands. Vaccine manufacturers prescribe booster doses to maintain protection: the first three years after primary immunization and subsequent boosters every three to five years. Sen Tai Bao is an exception, requiring an annual booster dose.17,19,85 In addition to conventional schemes, rapid vaccination schedules are available for most of these vaccines. If necessary, European vaccines can be used interchangeably.19
Although TBE vaccination is common in Europe, recommendations for TBE vaccination vary even among countries where TBE is endemic.1,9,67 At present, only Austria and Switzerland have national universal vaccination programs.1 In the Czech Republic, Estonia, Germany, Hungary, Latvia, Lithuania, and Slovenia, vaccination is generally recommended. Other European countries link their vaccine recommendations to specific factors, such as predefined risk areas. For example, in Croatia, Poland, and Serbia, vaccination is recommended for people living in or travelling to endemic areas. In Belarus, Italy, Kazakhstan, Mongolia, Slovakia, Sweden, Russia, and Ukraine, vaccination is recommended for those with possible occupational exposure. Several countries, including Belgium, Bulgaria, Finland, France, Greece, Ireland, Israel, Netherlands, Spain, UK, and Turkey, provide recommendations for individuals travelling to endemic regions.9 Simplifying vaccine recommendations could aid the public in understanding local guidelines.1
Although most countries require documentation of TBE-endemic areas in order to make targeted vaccination recommendations,1,32,33 it is unclear how national vaccination recommendations relate to observed TBE incidence, as incidence surveillance systems may underreport cases.9 The unpredictability of TBEV microfoci and the difficulty in identifying TBE-endemic areas raise questions about the suitability of vaccine recommendations that focus solely on these areas. Therefore, it may be advisable to expand TBE vaccine recommendations to cover the entire population, rather than just those residing in or travelling to currently identified endemic areas.1
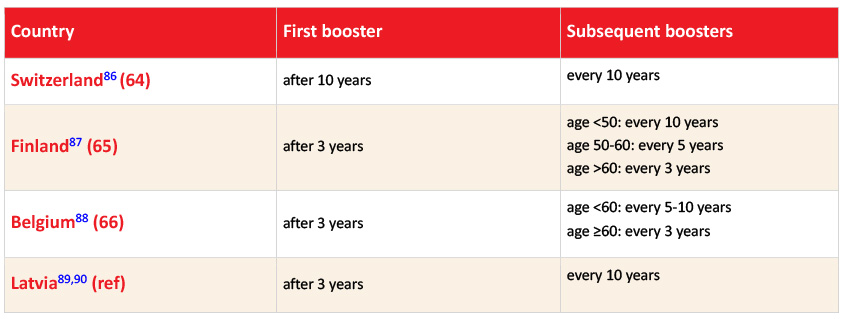
Regarding booster vaccinations, some countries, such as Switzerland, Finland, Belgium, and Latvia, have extended the recommended interval from every 3-5 years to up to 10 years, as approved locally (Table 2).86–90
Table 2: Booster dosing schedules in adults in Switzerland, Finland, and Belgium. Adapted from Schelling et al, 202491
In 2006, the Federal Office for Public Health in Switzerland recommended extending the booster intervals for TBE vaccine from 3 to 10 years. TBE vaccine reluctance was associated with the need for frequent boosters.92 After adjusting the vaccination schedule, the sales of the annual TBE vaccine increased more than four times,93 and vaccination coverage (1 dose) among children aged 16 increased from 10% (95% CI: 8.8-11.2%) in 2005-07 to 55% (95% CI: 53.0-56.6%) in 2020-22.94 In adults, the vaccination coverage reached 42% in 2018 (up to 50% in endemic regions).68 The Swiss strategy has not only been more cost-effective but has also led to a significant increase in the number of people accepting TBE vaccination without an increased rate of vaccine breakthrough infections in any age group, which is a substantial benefit for public health.95
TBE vaccination is fully or partially reimbursed in only a few countries. Typically, reimbursement is linked to specific factors.1,9 For example, Switzerland and Germany provide reimbursement for individuals who are travelling to, living in, or working in risk areas. Hungary provides reimbursement for residents of highly endemic areas, and Latvia provides partial reimbursement for children and adolescents living in endemic areas. In Austria, designated risk groups receive full coverage for vaccination costs. In Estonia, Latvia, and Poland, employers fully reimburse vaccination expenses for their employees falling into high-risk categories. In Slovenia, compulsory insurance schemes facilitate reimbursement for high-risk workers. In the Czech Republic, there are contributions from preventive funds from health insurance companies. TBE endemic countries that do not offer reimbursement for the TBE vaccine include Sweden and Romania.9 The absence of a broad reimbursement policy may be a significant factor in low vaccine uptake,96 as discussed below.
Vaccine effectiveness and vaccine uptake
TBE vaccines are highly effective in preventing infection, disease, and other outcomes, including serious outcomes, regardless of age.20,21,68,79,96–104 Vaccine effectiveness ranges from at least 91.5% for receipt of three or more doses68 to at least 95.4%20 for receipt of four or more doses.96 Studies have reported minimal differences in vaccine effectiveness estimates between individuals who received their last dose ≤10 years ago and those who received it more than 10 years ago.79,96,102,104
The impact of vaccination on disease incidence was well-documented in Austria. Austria is unique among European countries in having implemented an annual, nationwide TBE awareness and vaccination campaign as early as 1981, targeting the entire population. The implementation of vaccination programs has led to a substantial reduction in the incidence of TBE cases. In Austria, the number of TBE cases has decreased by approximately 90% compared to the time before vaccination programs were introduced and when vaccination coverage was low (Figure 1).98
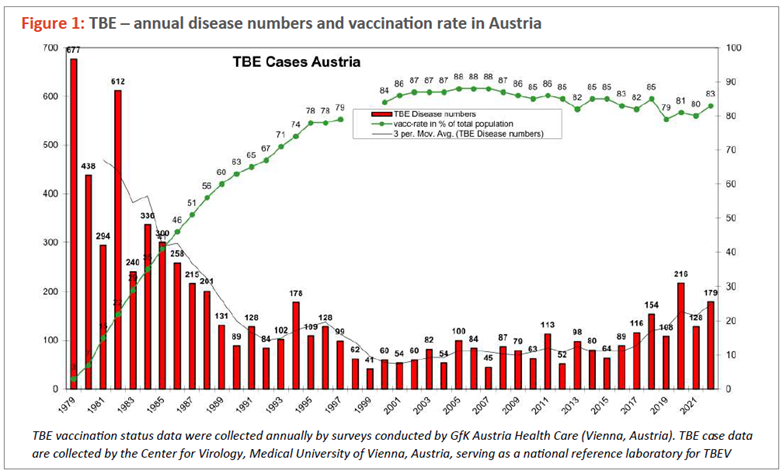
Between 2000 and 2011, TBE vaccination in Austria prevented approximately 333 cases annually within a population of 8.2 million.97 In Switzerland, TBE vaccination of adults was estimated to prevent 112-162 cases in 2018 among a population of 6.6 million adults.68 During the three-year study period in Latvia, vaccination was estimated to have prevented 897 hospitalizations, 26 intensive care admissions, 34 patients discharged from the hospital with paresis, and 20 deaths. Additionally, in the Czech Republic, TBE vaccination was estimated to prevent approximately 204 cases per year from 2018 to 2022 among a population of 10.4 million.105 Vaccination prevented over 1,000 cases of TBE and hundreds of hospitalizations annually in the four countries studied, highlighting the significant public health impact of TBE vaccines. These vaccines are widely used in over 25 European countries with TBE-endemic areas, suggesting that thousands of TBE cases are likely prevented each year through vaccination. However, even though TBE vaccines are effective, the incidence of TBE remains high in the endemic areas of many countries due to the high number of unvaccinated individuals.96
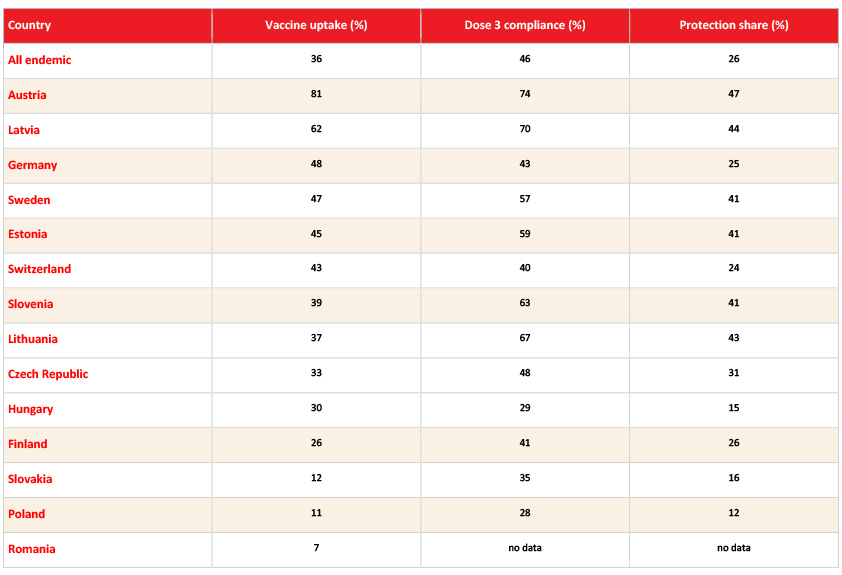
Uptake and compliance with TBE vaccination in Europe vary greatly, with overall low rates.1,67,106 The average TBE vaccine uptake in European countries was only 22% in endemic countries and 5% in non-endemic countries in 2020.67 At the country level, TBE vaccine coverage varies widely in endemic countries. Austria has the highest coverage at 81%, followed by Latvia at 62%. In contrast, Finland and Hungary have coverage of just under one-third of the population, while Slovakia, Poland, and Romania have the lowest coverage at 12%, 11%, and 7%, respectively (Table 3). In non-endemic countries, TBE vaccine coverage is very low, with only 1% in France, 5% in Belgium, 6% in the Netherlands, 7% in Norway, and 8% in Denmark and the United Kingdom.67
Table 3: Vaccine uptake in endemic European countries.67
“Vaccine uptake” was defined as the percentage of subjects with at least 1 TBE vaccination at any time. “Dose 3 compliance” measured the percentage of subjects who completed the primary series on time according to the licensed vaccine regimen after receiving their first dose of vaccine. “Protection share” measured the percentage of subjects who were within the licensed vaccination regimen after receiving at least 3 prior TBE vaccinations.
Click the image above to enlarge
In Russia, vaccination coverage varies greatly between regions, as reviewed in.19 The Rospotrebnadzor regulations prescribe mandatory vaccination of adolescents (at school) and high-risk groups in endemic territories, which is funded from the regional budget. In certain endemic areas, vaccination coverage can be high (e.g., 88% in the Sverdlovsk region). However, in other endemic regions, less than 10% of the population is vaccinated. The differences arise because vaccination is administered in endemic districts, while the level of vaccine coverage is calculated for the entire region. In non-endemic areas, vaccination is not compulsory, making it challenging to assess the impact of vaccination.19
In certain countries, high levels of disease and vaccine awareness may result in high vaccination rates, as seen in Austria. However, in other countries like the Czech Republic, despite high levels of awareness, vaccination rates remain low.67 In fact, vaccine uptake is a multifaceted issue that does not always correspond with vaccine awareness.1,67 The low vaccination rates across most of Europe can be attributed to various factors, including the complexity of the TBE vaccination schedule, low awareness of the potential consequences of TBE, and limited vaccine accessibility and reimbursement.106
The limited reimbursement of vaccine costs may reduce vaccine uptake due to economic constraints. For example, in Slovakia and Poland, the proportion of individuals who receive the vaccine is approximately five times smaller than the proportion of individuals who are aware of the availability of a TBE vaccine (12% vs. 63% in Slovakia, and 11% vs. 47% in Poland, respectively).67 In these countries, vaccination is (partially) reimbursed for high-risk occupational groups only.9 In contrast, in countries like Switzerland and Germany where the TBE vaccine is fully reimbursed for individuals staying in endemic areas, a high proportion of people who are aware of the vaccine’s availability actually get vaccinated9 (43% vs 59% in Switzerland, and 48% vs. 55% in Germany, respectively).67 Reimbursement can therefore be an important motivator for individuals to be vaccinated, and the introduction of a broad reimbursement policy can support better vaccine uptake. It is noteworthy, however, that despite the availability of low-cost TBE vaccines, their uptake remains low in some endemic countries due to limited awareness of the burden of the disease and the risk it poses.96 Thus, the relationship between vaccine uptake and reimbursement is not linear. It is influenced by other factors, as described above.
Necessity of pediatric vaccination
TBE vaccination is safe and effective and is currently recommended by the WHO for children one year of age and older.20,96,107–109 Seroconversion rates in children (up to 15 years of age based on data from clinical development programs) are similar to those in adults, approaching 100% even in children as young as 1 year of age.110–112 Studies have also shown high levels of protection and antibody persistence (94-100% seropositivity), with protection lasting up to 5 years following primary vaccination with three doses.113,114 A recent case-control study showed that TBE vaccination is highly effective (>90%) in fully vaccinated children 0-17 years in Switzerland and remains high for up to 10+ years post completion of primary vaccination.107
Despite evidence that the TBE vaccines used in Europe are both effective and safe, they are administered conservatively in children. Disease incidence is lower in children than in adults.14,24,70,71,115 However, infection in children may be underreported because symptoms are non-specific and vague, and children may not be able to describe their symptoms.25,72 About 40-80% of the children can recall tick-bites.25,116–118 In a study of asymptomatic TBE infections in a highly endemic area of northern Poland, only 2% of 180 unvaccinated children were seropositive for TBE, compared with 5% of adults, suggesting that TBE infections may be undiagnosed.119
The clinical course of TBE infection in children is similar to that in adults, albeit less severe. Although the frequency of occurrence varies, non-specific symptoms usually include fever, fatigue/malaise, behavioral changes, photophobia, myalgias.120 The most common clinical manifestation of the disease in children is meningitis in 60-80% of the cases, followed by 20-40% meningoencephalitis and 0-4% meningoencephalomyelitis.121,122 Disease severity is lower in children than in adults, but this discrepancy varies across the different age groups (0-5, 6-11 and 12-17 years).107 The biphasic clinical course typical of TBE infection is less common in pre-school children than in older children and adults.25 Consistent with the reduced overall incidence and severity of disease, permanent neurological sequelae of TBE infection are less common in children (0-2%)115,123–126 than in adults (30-50%).127–132 In a study of 523 TBE patients in Germany, overall 95% of 59 children and 64% of 464 adults recovered completely; compared with adults aged 18-39 years, the recovery rate in children was 79% higher.72 Post-encephalitic syndrome is reported 3-10 times more frequently in adults than in children, regardless of the severity of TBE and the time point during the 18-month follow-up.72
A comprehensive systematic review focusing on the epidemiology, clinical characteristics, and outcomes of TBE in the pediatric population confirmed that the disease is less severe in children. However, recent follow-up cases have shown that a significant proportion of children suffered from long-term cognitive impairment.24 These recent studies evaluating cognitive function in recovered pediatric TBE patients found abnormal EEG and MRI findings, a higher incidence of headache, fatigue, cognitive impairment, and reduced motor function compared to controls.118,125,133–135 Thus, although mild in the early stages, infections can lead to long-term neurological sequelae and increased morbidity in children, which can affect their performances in school and everyday life.72,118,122
A recent study in Switzerland evaluated 463 TBE cases in children aged 0-17 years.107 The study found that diagnoses of disease severity in young children aged 0-6 years are not different from those in older children. More severe disease, such as meningoencephalomyelitis, encephalomyelitis, and radiculitis, occurred in 1-5% of children across all three age groups (0-5, 6-11 and 12-17 years). The study also found that unvaccinated children were 6.7 times more likely than vaccinated children (1 or more doses) to develop neurological disease symptoms. Incompletely vaccinated children (2 doses or less) and completely vaccinated children (3 or more doses) were less likely to experience mild neurological disease compared to unvaccinated children.
Given the recent increase in incidence and severity of TBE, it is important to improve vaccination rates among children and adolescents. As they are more likely to engage in outdoor activities, children are at high risk, particularly those between 5 and 14 years.12,64,66 Among the factors associated with uptake of TBE vaccination, having had a recent tick bite was the only predictor of having had a child vaccinated against TBE.119 As TBE cases in children may be underreported, and mild symptoms may develop into long-term cognitive impairment, vaccination should be encouraged for children, especially those living in or travelling to TBE-endemic area.
TBE vaccination and travel
Global incidence estimates of TBE range from 10,000 to 12,000 cases per year,109 with many cases remaining unreported or misdiagnosed.1 According to the United Nations World Tourism Organization, there were almost 1.3 billion international tourist arrivals in 2023, which represents an increase of 34% from 2022.136 More than half of these arrivals occurred in Europe.136 The increase in international tourism, particularly in Europe, increases the risk of individuals travelling from non-endemic to endemic TBE areas.137,138
While the risk of mortality due to TBE is relatively low (ranging from 1% in central Europe up to 40% in the Far East),139 the burden of long-term morbidity can be significant, lasting from months to years and ranging from post-encephalitic syndrome to permanent paralysis and seizures.140 As there is currently no specific treatment for TBE, prevention is recommended. This includes preventing tick-bites, as described earlier, and vaccination. TBE vaccines may be administered in an accelerated schedule shortly before travel.96,108 Vaccination is recommended for travellers from non-endemic countries with a high risk of tick exposure during travel between April and November.138,141 Therefore, it is important to assess the risk of acquiring TBE for travellers from non-endemic countries visiting endemic countries before deciding whether to get vaccinated. This assessment should consider both environmental and personal factors. Environmental concerns relate to the choice of destination, including whether the area is endemic for TBE, the season, and altitude. Surveillance data have shown that tick activity is highest between April and November in endemic areas,12,142 and TBEV foci have been found in places as high as 2100 meters above sea level.143 When assessing the risk of exposure, it is important to consider individual behavior, the type of outdoor activity, duration of stay, and demographic variables such as age, gender, and personal health status.140
Several studies have assessed awareness of TBE and the TBE vaccine among travellers.78,144,145 One study assessed perceptions of TBE risks among travellers from Canada, Germany, Sweden and the United Kingdom who were travelling to a TBE-endemic country.144 The study found that 69% of travellers were aware of the disease, and 26% prepared for their trip by searching for information online. While 14% were aware that TBE vaccines were offered by travel clinics, 52% were not aware of the existence of travel clinics. Furthermore, while 14% of participants reported feeling at high risk when travelling to an endemic region, 26% never felt at risk. Among those who engaged in pre-defined at-risk activities, such as camping or hiking in the forests, 79% were aware of at least one correct TBE prevention measure. However, only 15% had been vaccinated within the last 3 years and 11% had been vaccinated following a clinic recommendation. Only 35% of the participants had heard of a TBE vaccine. Health professionals working in travel clinics recommended TBE vaccination to 61% of their travellers going to endemic areas.144 Another study that surveyed international travellers residing in the United States found that the likelihood of travellers choosing the TBE vaccine depends on the level of endemic risk in the destination country, provided that the vaccine is available at no cost.145 Almost all travellers (94%) would choose to be vaccinated should the risk be at the highest level, whereas 6% would remain unvaccinated regardless of the risk level. Respondents who participated in outdoor activities were more likely to choose vaccination than the average respondent.
While TBE awareness may have increased among travellers and travel clinics, vaccination may not be available in the country of origin where TBE is not endemic. Additionally, the subsequent costs of vaccination, diagnosis, and medical care may not be covered. If symptoms of infection occur upon returning home, they may not be recognized, leading to a misdiagnosis or no diagnosis at all, especially if adequate diagnostic testing tools are not available.146
In conclusion, it is important for both travellers and health professionals in travel clinics to be well-informed about the risks, preventive measures and symptoms of TBE when travelling from a TBE non-endemic country to an endemic destination. Lack of awareness or failure to take the necessary precautions could increase the likelihood of infection. These concerns highlight the need for international guidelines on TBE for travellers.
Economic impact
Health economic evaluations inform medical procurement and reimbursement decisions by public and private healthcare providers. The most common form of health economic evaluation is cost-effectiveness analysis, which presents the ratio of the incremental cost of an intervention to the incremental health benefits of an intervention.147 However, there are only a few cost-effectiveness evaluations of the TBE vaccine.
In 1981, Austria introduced an overall TBE vaccination campaign97 that led to a significant reduction in TBE cases.99 The economic benefit of the campaign, which included reducing costs for inpatient care, loss of productivity, and premature retirement, was evaluated to be EUR 24 million for the years 1981 to 1990148 and EUR 60 million between 1991 and 2000.
A study conducted in Slovenia found that TBE vaccination is cost-effective from a healthcare payer’s perspective when vaccination begins at 18 years of age and continues until the age of 80.149
In 1996, a cost-effectiveness estimation of TBE vaccination in the Stockholm area was performed and it was calculated that, based on the TBE incidence at that time and the cost of vaccination, mass vaccination would be an unrealistic alternative.150 However, more than 20 years later, much higher incidences in the unvaccinated population were reported. A health economic analysis was conducted in Sörmland County, which is a highly TBE-endemic area adjacent to Stockholm County. The analysis calculated that the costs per QALY (quality adjusted life year) for a fully free-of-charge vaccination program would come much closer to the generally acceptable cost-effectiveness threshold in Sweden. The authors concluded that introducing a structured vaccination program would be cost-effective at all ages. However, it would be particularly cost-effective if implemented in childhood.77
Estimating the economic impact of a disease requires an assessment of its disease burden, in addition to cost-benefit analyses. The Burden of Communicable Diseases in Europe study computed disability-adjusted life years (DALYs) for 31 selected diseases, including tick-borne encephalitis, in the European Union and European Economic Area.151 DALYs represent the equivalent of a year of full health lost and are the sum of the years of life lost due to premature mortality and the years lived with a disability. The calculation of DALYs relies on the incidence of acute, symptomatic disease as a crucial input variable. Furthermore, it requires several age-group and sex-specific variables, such as the risk of developing short- and long-term complications, their duration, and weights reflecting their severity. The study found that the median annual burden of TBE was 0.69 (0.65-0.74) DALYs per 100,000 population.151 It is worth noting that a Slovenian study found a much higher disease burden on the country level (11.0 (10.2-11.7) per 100,000).152 Thus, differences in underlying assumptions and disease modelling approaches heavily influence the outcomes of such analyses. Although DALYs provide useful information for prioritization and planning in public health, they do not fully encompass all unknowns, uncertainties, variability and other “softer” criteria such as public perception.153
A TBE vaccination program must be evaluated against other healthcare resources. To determine if funding a TBE vaccination program yields better health outcomes at a reasonable cost, it is important to establish the long-term costs and health outcomes of a local TBE vaccination strategy.154 Furthermore, TBE can result in high productivity loss beyond the healthcare sector. Increasing vaccination rates across all age groups is the most effective and efficient strategy to reduce the burden of TBE and protect the overall population’s health.155 Therefore, a vaccination program or at least a vaccination recommendation should be considered. It is important to note that out-of-pocket costs may have a positive impact on an individual’s private consumption, which is not included in the health care analysis.
Health economic evaluations play a crucial role in informing decisions regarding the implementation of TBE vaccination programs. While the cost-effectiveness of such programs varies depending on factors such as incidence rates and population demographics, evidence suggests that TBE vaccination can yield significant economic benefits by reducing healthcare costs and productivity losses. Despite challenges in estimating disease burden and modelling economic impacts, prioritizing TBE vaccination efforts across age groups remains a cost-effective strategy for mitigating the overall burden of the disease and safeguarding public health.
The One Health approach
The One Health approach is a collaborative and holistic strategy that recognizes the interconnectedness of human, animal, and environmental health.156 TBE involves a complex ecosystem in which the virus circulates between ticks, animals (such as small mammals and deer), and humans.157–159 The One Health approach considers the interdependence of these systems with the environment and seeks to understand how changes in one component can affect the entire ecosystem.
As discussed earlier in this chapter, the tick species Ixodes ricinus is the predominant TBEV vector in Europe, while Ixodes persulcatus and Haemaphysalis concinna are found in Russia and Asia.158,160 The main reservoir hosts for ticks are small mammals or insectivores such as rodents, hedgehogs, shrews and hares. While their small size makes them easy targets for ticks, especially nymphs, their biological characteristics allow TBEV to circulate in the bloodstream at levels that allow the virus to be transmitted to feeding ticks without killing them. As these hosts have a high reproductive rate and short lifespan, there are always enough animals naive to the virus for it to spread.158 Larger animals, such as deer, serve as hosts for adult ticks.161 With a lag time of one year, a study in Sweden showed that the number of roe deer and hares was positively correlated with the number of TBE cases in the region.162
Tick populations are also strongly influenced by environmental factors such as climate, vegetation, habitat and human activity.157 As discussed earlier, climate change can influence the survival, abundance and activity of ticks and their hosts by affecting the vegetation and their habitat through prolonged higher temperatures and relative humidity.35–38 Human activity has also changed over the years, which has contributed to the increase in TBE cases. In addition to heightened awareness of the diagnosis of the disease, the number of TBE cases could be affected by farming and global tourism (both recreational and business). This increases the possibility of human and tick contact when individuals travel from a non-TBE endemic region to a TBE endemic region.1,35–38,137,138,157 Surveillance of tick, animal, and human activities can aid in tracking the prevalence of TBEV, identifying potential hotspots, assessing the risk of human exposure, and exploring the dynamics of cross-species transmission to reduce the risk of spillover events.
A model incorporating data on climate, forest cover, water, tick abundance, and sheep (as an indicator species) identified an increase in TBE incidence in the Örebro region of Sweden during the study period.163 They found a variation in hotspots across the region. The risk of acquiring TBE increased by 12.5% for every 1% increase in relative humidity and by 72.3% for every 1% increase in the proportion of wetland forest. However, as the model had a low goodness of fit, other variables, such as human behavior could help create a stronger model for understanding the spatial distribution of ticks. Historical data on TBE cases, human population demographics and migration, climate teleconnection, beech fructification (used as a proxy for rodent density, which acts as a host for the TBE virus vector), and annual sunshine duration were used to forecast TBE incidences for Austria, Germany, and Switzerland from 2019 to 2021.45 The first verified forecasting results for 2019 were highly reliable, but could be improved for better accuracy.164
The most common way to contract TBE is through a tick bite. However, it is also possible to acquire TBE through the consumption of unpasteurized TBEV-contaminated dairy products from goats, cows and sheep.61 The largest outbreak of TBE occurred in 1951 in the former Czechoslovakia, where over 600 cases were reported due to the consumption of contaminated, unpasteurized cow and goat milk.61,165 An analysis of TBE outbreaks in Slovakia from 2007 to 2016 revealed that 17% of all TBE cases were due to consumption of dairy products.166 This percentage showed an increasing linear trend throughout the study period. Notably, none of these cases reported a tick bite, nor were they vaccinated against TBE.166 A systematic review and meta-analysis of 410 cases of foodborne-TBE (FB-TBE) between 1980 and 2021 confirmed that the majority of cases were located in Central and Eastern Europe (the so-called FB-TBE triangle) and Russia.63 The clinical presentation is similar to non FB-TBE infections, and neuroinvasive disease is common in 39% of cases. However, the median incubation time is shorter at 3.5 days. None of the cases were vaccinated, except for one whose last booster was more than 15 years ago. The clinical attack rate was 14% in outbreaks with 10 or more cases, with significant heterogeneity.63 These FB-TBE outbreaks have the potential to cause a significant public health issue, despite their infrequency. However, unlike non FB-TBE cases, patients with mild and nonspecific symptoms can be actively contacted during an epidemiological investigation to locate the source of the outbreak. FB-TBE cases can be prevented by vaccination and avoidance of unpasteurized dairy products in TBE-endemic areas.100
In April 2020, the first FB-TBE outbreak occurred in France where the virus had never been detected before.167 The research team utilized the One Health approach to investigate the outbreak.159 Forty-two out of 43 cases of FB-TBE were linked to the consumption of unpasteurized raw goat cheese from a local producer. The methodology of investigation included screening for TBEV in cheese and milk products to identify the source of infection, serological testing of all animals on the suspected farm and surrounding farms, landscape analysis and localization of the wooded area, ticks, and small animal surveys for virus detection and virus isolation and genome sequencing. Information gained from this thorough and integrative approach should help the farmers and health authorities assess the risk of infection and develop control strategies. This outbreak underscored the need to improve surveillance, detection and prevention of FB-TBE in France, particularly given the increasing global trend toward the consumption of local and traditional delicacies.168
In summary, the One Health approach provides a robust framework for understanding and addressing the complexity of TBE. By acknowledging the interdependence of human, animal, and environmental health and involving health authorities and local communities, this collaborative strategy enables comprehensive surveillance, targeted interventions and effective control measures. Interdisciplinary collaboration and integrated surveillance systems are essential steps in reducing the burden of TBE and protecting public health.
Recommendations
TBE is considered an emerging disease and a growing public health concern. A One Health approach should be considered to combat this complex problem, as it emphasizes the importance of interdisciplinary collaboration in addressing complex health challenges by highlighting the interconnectedness of tick, human, animal, and environmental health.
Although there is considerable variation in national reporting of annual cases, the cumulative number of reported TBE cases across Europe is increasing, highlighting the need for improved TBE risk management. 1,9,10 Surveillance methods for TBE vary across Europe, with countries using different diagnostic criteria, access to diagnostic tests and knowledge of their appropriateness, and approaches to national and regional surveillance.1,9,13 Surveillance of TBE in Europe is currently incomplete, which means that reported cases are likely to only partially reflect the true risk, and that the true burden of TBE is significantly underestimated.1,9,67,106 Experts on TBE have suggested the following measures to improve the surveillance of TBE throughout Europe:1
- Use of a single TBE case definition across Europe to ensure comparability of data;
- Testing all cases of aseptic meningitis/encephalitis of unknown etiology for TBEV infection;
- Rapidly extend testing to all patients with either a fever of unknown origin or CNS symptoms who live in or have visited an endemic, probable, or potential endemic area or who have received a tick bite;
- Improved funding for and access to diagnostic tests and testing facilities;
- Establishment of nationwide surveillance systems in countries that do not have them by implementing active surveillance systems with interactive maps of Ixodid tick activity across Europe; and
- Implementing active surveillance systems throughout Europe.
The national TBE disease burden and funding constraints will largely determine the extent to which these measures are implemented.1
Other recommendations to address the challenges as outlined in this chapter include:
- Climate change: The influence of climate change on the transmission of tick-borne diseases includes its impact on the survival, abundance, and activity of ticks, as well as their hosts. Changes in temperature, precipitation, and vegetation are expected to alter the geographic distribution and prevalence of diseases like TBE.35–38 The spread of TBE to new regions in Europe presents a significant public health challenge. This challenge involves implementing measures to prevent TBE in regions not previously affected by the disease and where awareness of the disease is low. Such measures include establishing a surveillance system, recommending vaccination, and conducting awareness-raising campaigns.
- TBE vaccination recommendation: Vaccination remains the most effective method of protection against TBE. However, National Immunization Technical Advisory Groups in some European countries with TBE-endemic areas do not recommend TBE vaccines,96 and only a few European countries have universal vaccination recommendations.1,9 The unpredictability of TBEV microfoci and the difficulty in identifying TBE-endemic areas raise questions about the suitability of vaccine recommendations that focus solely on these areas. It may be advisable to expand TBE vaccine recommendations to cover the entire population, rather than just those residing in or travelling to currently identified endemic areas.1 Alternatively, if TBE risk is limited to specific areas or if vaccination poses a significant burden on national or local healthcare services, vaccine recommendations should be simplified and standardized for healthcare practitioners and the public. TBE experts believe that this will aid the public in comprehending the recommendations and minimizing confusion.1 In order for TBE vaccine recommendations to be effective, it is crucial that the public trusts the recommendations, understands the health risks associated with tick bites, has knowledge of TBE, and has easy access to vaccination services.1,67,169
- TBE vaccination rates: Uptake and compliance with TBE vaccination in Europe vary greatly, with overall low rates.1,67,106 The uptake of the TBE vaccine is influenced by various factors, including specific recommendations, public awareness programs, vaccine awareness, perceptions of vaccine safety and reimbursement.67 In many countries where TBE vaccines are recommended, vaccine uptake is low due to limited reimbursement of vaccine costs.96 Although some countries have achieved good levels of vaccine uptake without a comprehensive national program,67,106 vaccine reimbursement could lead to improved vaccine uptake, especially in low-income households.169 However, in some countries, TBE vaccines are recommended and available at low cost, but vaccine uptake remains inadequate due to limited awareness of the disease burden and understanding of the risk.96 Therefore, in countries where high awareness of the disease and vaccine does not directly translate into high vaccine uptake, motivators and barriers to vaccination must be analyzed to increase vaccine uptake. In countries where low vaccine awareness is associated with limited vaccine uptake, it is necessary to improve public awareness of TBE vaccines. In countries with low vaccine compliance, it is important to emphasize the need for booster shots.67
- TBE awareness and risk exposure: The incidence of TBE has increased over the past 25 years, posing a risk to individuals living in both TBE endemic and non-endemic countries, especially with the growth in international tourism.137,138,157 Although TBE mortality rates are low, long-term morbidity underscores the importance of prevention. Therefore, safe and effective TBE vaccination is strongly recommended for travellers from non-endemic areas with a high risk of tick exposure. Studies show that awareness of TBE varies among individuals.1,67,138,170 Therefore, comprehensive risk assessments that include environmental and personal factors are necessary. Targeted awareness campaigns and the involvement of health professionals are essential to promote preventive measures. These campaigns should focus on risk areas, risk perception, and the benefits of vaccination to address barriers and misconceptions. These campaigns should improve access to vaccination while tailoring interventions to specific populations, such as the elderly, immunocompromised individuals, individuals with comorbidities and behavioral and occupational risks, and travellers.
- TBE vaccination for children: Evidence strongly supports the safety and efficacy of TBE vaccination in children, with seropositivity comparable to adults and high long-term protection rates.24,107,108,110,111,113 However, despite its proven benefits, vaccination rates remain conservative, possibly due to lower disease incidence in children and underreporting of TBE cases. In recent years, there has also been an increase in cases of neurological sequelae and long-term cognitive impairment in children diagnosed with TBE.24,72,118,133 To address this, there should be a concerted effort to increase vaccination uptake among children and adolescents, particularly in endemic areas. Given the potential underreporting or missed diagnoses of TBE, particularly in preschool children, pediatricians in TBE-endemic regions should remain vigilant for TBEV infection in children presenting with non-specific central nervous system symptoms. It is imperative for them to ensure comprehensive clinical follow-up for children diagnosed with TBE to address potential long-term morbidity.
- Economic impact: Health economic evaluations are essential to guide decisions about the implementation of TBE vaccination programs. Despite the limited number of cost-effectiveness analyses of the TBE vaccine, studies have demonstrated its economic benefits, particularly in reducing healthcare costs and productivity losses. Evaluating the long-term costs and health outcomes of local vaccination strategies is essential to determine their effectiveness and prioritize resource allocation. Increasing vaccination coverage across all age groups has been identified as the most effective strategy for reducing the burden of TBE and protecting public health. Despite challenges in estimating disease burden and economic impact, prioritizing TBE vaccination efforts is considered cost-effective and essential to reduce the overall burden of the disease.
Contacts
Rahel Ackermann-Gäumann
rahel.ackermann@ne.ch
Authors
Phung Lang, Rahel Ackermann-Gäumann
Citation
Lang P, Ackermann-Gäumann R. Public health aspects of TBE.
Chapter 14. In: Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ, eds. The TBE Book.7th ed. Singapore: Global Health Press; 2024. 10.33442/26613980_14-7
References
- Kunze M, Banović P, Bogovič P, et al. Recommendations to Improve Tick-Borne Encephalitis Surveillance and Vaccine Uptake in Europe. Microorganisms. 2022;10(7):1283. doi:10.3390/microorganisms10071283
- Rosicky B. Notes on the classification of natural foci of tick-borne encephalitis in Central and South-East Europe. J Hyg Epidemiol Microbiol Immunol. 1959;3:431-443.
- Blaskovic D, Nosek J. The ecological approach to the study of tick-borne encephalitis. Prog Med Virol. 1972;14:275-320.
- Dobler G, Erber W, Bröker M, Chitimia-Dobler L, Schmitt HJ. Global distribution of the TBEV. Chapter 12c. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. Global Health Press; 2023.
- Centers for Disease Control and Prevention. Tick-borne Diseases Abroad. Published 2022. Accessed February 7, 2024. https://www.cdc.gov/ticks/tickbornediseases/abroad.html
- Im JH, Baek JH, Durey A, Kwon HY, Chung MH, Lee JS. Geographic distribution of Tick-borne encephalitis virus complex. J Vector Borne Dis. 2020;57(1):14-22. doi:10.4103/0972-9062.308794
- Jenkins VA, Silbernagl G, Baer LR, Hoet B. The epidemiology of infectious diseases in Europe in 2020 versus 2017–2019 and the rise of tick-borne encephalitis (1995–2020). Ticks and Tick-borne Diseases. 2022;13(5):101972. doi:10.1016/j.ttbdis.2022.101972
- Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020;27(8). doi:10.1093/jtm/taaa180
- Erber W, Schmitt HJ, Jankovic TV. TBE-epidemiology by country—an overview. Chapter 12a. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. Global Health Press; 2023. doi:10.33442/26613980_12a-6
- Daniel M, Danielová V, Kríz B, Jirsa A, Nozicka J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in central Europe. Eur J Clin Microbiol Infect Dis. 2003;22(5):327-328. doi:10.1007/s10096-003-0918-2
- Van Heuverswyn J, Hallmaier-Wacker LK, Beauté J, et al. Spatiotemporal spread of tick-borne encephalitis in the EU/EEA, 2012 to 2020. Euro Surveill. 2023;28(11). doi:10.2807/1560-7917.Es.2023.28.11.2200543
- European Centre for Disease Prevention and Control ECDC. Tick-borne encephalitis Annual Epidemiological Report for 2020. Published online 2022. Accessed December 29, 2023.
- European Centre for Disease Prevention and Control ECDC. Surveillance Systems Overview for 2022. Available Online: Https://Www.Ecdc.Europa.Eu/En/Publications-Data/Surveillance-Systems-Overview-2022. Accessed December 29, 2023.
- European Centre for Disease Prevention and Control (ECDC). EU Case Definitions.(2018). Accessed December 29, 2023. https://www.ecdc.europa.eu/en/all-topics/eu-case-definitions
- Hellenbrand W, Kreusch T, Böhmer MM, et al. Epidemiology of Tick-Borne Encephalitis (TBE) in Germany, 2001−2018. Pathogens. 2019;8(2). doi:10.3390/pathogens8020042
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother. 2013;9(2):362-374. doi:10.4161/hv.22766
- Kollaritsch H, Paulke-Korinek M, Holzmann H, Hombach J, Bjorvatn B, Barrett A. Vaccines and vaccination against tick-borne encephalitis. Expert Rev Vaccines. 2012;11(9):1103-1119. doi:10.1586/erv.12.86
- Bogovic P, Lotric-Furlan S, Strle F. What tick-borne encephalitis may look like: clinical signs and symptoms. Travel Med Infect Dis. 2010;8(4):246-250. doi:10.1016/j.tmaid.2010.05.011
- Ruzek D, Avsic Zupanc T, Borde J, et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res. 2019;164:23-51. doi:10.1016/j.antiviral.2019.01.014
- Erber W, Khan F, Zavadska D, et al. Effectiveness of TBE vaccination in southern Germany and Latvia. Vaccine. 2022;40(5):819-825. doi:10.1016/j.vaccine.2021.12.028
- Santonja I, Stiasny K, Essl A, Heinz FX, Kundi M, Holzmann H. Tick-Borne Encephalitis in Vaccinated Patients: A Retrospective Case-Control Study and Analysis of Vaccination Field Effectiveness in Austria From 2000 to 2018. J Infect Dis. 2023;227(4):512-521. doi:10.1093/infdis/jiac075
- Zavadska D, Odzelevica Z, Karelis G, et al. Tick-borne encephalitis: A 43-year summary of epidemiological and clinical data from Latvia (1973 to 2016). PLoS One. 2018;13(11):e0204844. doi:10.1371/journal.pone.0204844
- Bogovič P, Kastrin A, Lotrič-Furlan S, et al. Clinical and Laboratory Characteristics and Outcome of Illness Caused by Tick-Borne Encephalitis Virus without Central Nervous System Involvement. Emerg Infect Dis. 2022;28(2):291-301. doi:10.3201/eid2802.211661
- Steffen R. Tick-borne encephalitis (TBE) in children in Europe: Epidemiology, clinical outcome and comparison of vaccination recommendations. Ticks and Tick-borne Diseases. 2019;10(1):100-110. doi:10.1016/j.ttbdis.2018.08.003
- Hansson MEA, Örvell C, Engman ML, et al. Tick-Borne Encephalitis in Childhood: Rare or Missed? The Pediatric Infectious Disease Journal. 2011;30(4):355. doi:10.1097/INF.0b013e3181fe3b5a
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21 Suppl 1:S36-40. doi:10.1016/s0264-410x(02)00819-8
- Lindquist L. Tick-borne encephalitis. In: Tselis AC, Booss J, eds. Handbook of Clinical Neurology. Vol 123. Elsevier B.V.; 2014.
- Banović P, Obregón D, Mijatović D, et al. Tick-Borne Encephalitis Virus Seropositivity among Tick Infested Individuals in Serbia. Pathogens. 2021;10(3). doi:10.3390/pathogens10030301
- Yoshii K, Ikawa A, Chiba Y, et al. Establishment of a neutralization test involving reporter gene-expressing virus-like particles of tick-borne encephalitis virus. Journal of Virological Methods. 2009;161(1):173-176. doi:10.1016/j.jviromet.2009.05.016
- Haviernik J, Eyer L, Yoshii K, et al. Development and characterization of recombinant tick-borne encephalitis virus expressing mCherry reporter protein: A new tool for high-throughput screening of antiviral compounds, and neutralizing antibody assays. Antiviral Res. 2021;185:104968. doi:10.1016/j.antiviral.2020.104968
- Domanovic D, Giesecke J. How to define an area where transmission of arthropod-borne disease is occurring? Euro Surveill. 2012;17(20).
- Stefanoff P, Polkowska A, Giambi C, et al. Reliable surveillance of tick-borne encephalitis in European countries is necessary to improve the quality of vaccine recommendations. Vaccine. 2011;29(6):1283-1288. doi:10.1016/j.vaccine.2010.11.077
- Braks M, van der Giessen J, Kretzschmar M, et al. Towards an integrated approach in surveillance of vector-borne diseases in Europe. Parasit Vectors. 2011;4:192. doi:10.1186/1756-3305-4-192
- Stefanoff P, Zielicka-Hardy A, Hlebowicz M, et al. New endemic foci of tick-borne encephalitis (TBE) identified in districts where testing for TBE was not available before 2009 in Poland. Parasit Vectors. 2013;6:180. doi:10.1186/1756-3305-6-180
- Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:593232. doi:10.1155/2009/593232
- Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. N Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019;45(4):83-89. doi:10.14745/ccdr.v45i04a02
- Randolph SE. [Fauna, climate and politics: possible causes for the recent increases in tick-borne zoonoses]. Arch Pediatr. 2004;11(10):1282-1285. doi:10.1016/j.arcped.2003.12.019
- Randolph SE. Is expert opinion enough? A critical assessment of the evidence for potential impacts of climate change on tick-borne diseases. Anim Health Res Rev. 2013;14(2):133-137. doi:10.1017/S1466252313000091
- Bouchard C, Aenishaenslin C, Rees EE, et al. Integrated Social-Behavioral and Ecological Risk Maps to Prioritize Local Public Health Responses to Lyme Disease. Environ Health Perspect. 2018;126(4):047008. doi:10.1289/ehp1943
- Aenishaenslin C, Bouchard C, Koffi JK, Ogden NH. Exposure and preventive behaviours toward ticks and Lyme disease in Canada: Results from a first national survey. Ticks Tick Borne Dis. 2017;8(1):112-118. doi:10.1016/j.ttbdis.2016.10.006
- Aenishaenslin C, Bouchard C, Koffi JK, Pelcat Y, Ogden NH. Evidence of rapid changes in Lyme disease awareness in Canada. Ticks Tick Borne Dis. 2016;7(6):1067-1074. doi:10.1016/j.ttbdis.2016.09.007
- Gray JS. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. International Journal of Medical Microbiology. 2008;298:19-24. doi:10.1016/j.ijmm.2007.09.005
- Brugger K, Walter M, Chitimia-Dobler L, Dobler G, Rubel F. Seasonal cycles of the TBE and Lyme borreliosis vector Ixodes ricinus modelled with time-lagged and interval-averaged predictors. Exp Appl Acarol. 2017;73(3-4):439-450. doi:10.1007/s10493-017-0197-8
- Tkadlec E, Václavík T, Široký P. Rodent Host Abundance and Climate Variability as Predictors of Tickborne Disease Risk 1 Year in Advance. Emerg Infect Dis. 2019;25(9):1738-1741. doi:10.3201/eid2509.190684
- Rubel F, Walter M, Vogelgesang JR, Brugger K. Tick-borne encephalitis (TBE) cases are not random: explaining trend, low- and high-frequency oscillations based on the Austrian TBE time series. BMC Infect Dis. 2020;20(1):448. doi:10.1186/s12879-020-05156-7
- Marini G, Tagliapietra V, Cristofolini F, et al. Correlation between airborne pollen data and the risk of tick-borne encephalitis in northern Italy. Sci Rep. 2023;13(1):8262. doi:10.1038/s41598-023-35478-w
- Bogdziewicz M, Kelly D, Thomas PA, Lageard JGA, Hacket-Pain A. Climate warming disrupts mast seeding and its fitness benefits in European beech. Nat Plants. 2020;6(2):88-94. doi:10.1038/s41477-020-0592-8
- Bouchard C, Leighton PA, Beauchamp G, et al. Harvested white-tailed deer as sentinel hosts for early establishing Ixodes scapularis populations and risk from vector-borne zoonoses in southeastern Canada. J Med Entomol. 2013;50(2):384-393. doi:10.1603/me12093
- Levi T, Kilpatrick AM, Mangel M, Wilmers CC. Deer, predators, and the emergence of Lyme disease. Proc Natl Acad Sci U S A. 2012;109(27):10942-10947. doi:10.1073/pnas.1204536109
- Roy-Dufresne E, Logan T, Simon JA, Chmura GL, Millien V. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: implications for the spread of lyme disease. PLoS One. 2013;8(11):e80724. doi:10.1371/journal.pone.0080724
- Simon JA, Marrotte RR, Desrosiers N, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol Appl. 2014;7(7):750-764. doi:10.1111/eva.12165
- Kilpatrick AM, Dobson ADM, Levi T, et al. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc Lond B Biol Sci. 2017;372(1722). doi:10.1098/rstb.2016.0117
- Elväng A, Melik W, Bertrand Y, Lönn M, Johansson M. Sequencing of a tick-borne encephalitis virus from Ixodes ricinus reveals a thermosensitive RNA switch significant for virus propagation in ectothermic arthropods. Vector Borne Zoonotic Dis. 2011;11(6):649-658. doi:10.1089/vbz.2010.0105
- Friedsam AM, Brady OJ, Pilic A, Dobler G, Hellenbrand W, Nygren TM. Geo-Spatial Characteristics of 567 Places of Tick-Borne Encephalitis Infection in Southern Germany, 2018-2020. Microorganisms. 2022;10(3). doi:10.3390/microorganisms10030643
- Alkishe AA, Peterson AT, Samy AM. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One. 2017;12(12):e0189092. doi:10.1371/journal.pone.0189092
- Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;5:8. doi:10.1186/1756-3305-5-8
- Heinz FX, Stiasny K, Holzmann H, et al. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Eurosurveillance. 2015;20(13):21077. doi:10.2807/1560-7917.ES2015.20.13.21077
- Beermann S, Dobler G, Faber M, et al. Impact of climate change on vector- and rodent-borne infectious diseases. J Health Monit. 2023;8(Suppl 3):33-61. doi:10.25646/11401
- Walter M, Brugger K, Rubel F. The ecological niche of Dermacentor marginatus in Germany. Parasitol Res. 2016;115(6):2165-2174. doi:10.1007/s00436-016-4958-9
- Kunze M, Erber W, Haditsch M. TBE as a matter of public health.Chapter 13. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Global Health Press, Singapore, 2023. doi: 10.33442/26613980_13-6
- Ruzek D, Kaucka K. A brief tale of two pioneering moments: Europe’s first discovery of Tick-Borne Encephalitis (TBE) virus beyond the Soviet Union and the largest alimentary TBE outbreak in history. Ticks Tick Borne Dis. 2024;15(3):102314. doi:10.1016/j.ttbdis.2024.102314
- Buczek AM, Buczek W, Buczek A, Wysokińska-Miszczuk J. Food-Borne Transmission of Tick-Borne Encephalitis Virus—Spread, Consequences, and Prophylaxis. Int J Environ Res Public Health. 2022;19(3):1812. doi:10.3390/ijerph19031812
- Elbaz M, Gadoth A, Shepshelovich D, Shasha D, Rudoler N, Paran Y. Systematic Review and Meta-analysis of Foodborne Tick-Borne Encephalitis, Europe, 1980-2021. Emerg Infect Dis. 2022;28(10):1945-1954. doi:10.3201/eid2810.220498
- Zimmermann H, Koch D. [Epidemiology of tick-borne encephalitis (TBE) in Switzerland 1984 to 2004]. Ther Umsch. 2005;62(11):719-725. doi:10.1024/0040-5930.62.11.719
- Altpeter E, Zimmermann H, Oberreich J, Péter O, Dvořák C, Network SSS. Tick related diseases in Switzerland, 2008 to 2011. Swiss Medical Weekly. 2013;143(0102):w13725-w13725. doi:10.4414/smw.2013.13725
- Schuler M, Zimmermann H, Altpeter E, Heininger U. Epidemiology of tick-borne encephalitis in Switzerland, 2005 to 2011. Eurosurveillance. 2014;19(13). doi:10.2807/1560-7917.ES2014.19.13.20756
- Pilz A, Erber W, Schmitt HJ. Vaccine uptake in 20 countries in Europe 2020: Focus on tick-borne encephalitis (TBE). Ticks and Tick-borne Diseases. 2023;14(1):102059. doi:10.1016/j.ttbdis.2022.102059
- Baroutsou V, Zens KD, Sinniger P, Fehr J, Lang P. Analysis of Tick-borne Encephalitis vaccination coverage and compliance in adults in Switzerland, 2018. Vaccine. 2020;38(49):7825-7833. doi:10.1016/j.vaccine.2020.10.022
- Krawczuk K, Czupryna P, Pancewicz S, Ołdak E, Moniuszko-Malinowska A. Comparison of tick-borne encephalitis between children and adults—analysis of 669 patients. J Neurovirol. 2020;26(4):565-571. doi:10.1007/s13365-020-00856-x
- Kohlmaier B, Schweintzger NA, Sagmeister MG, et al. Clinical Characteristics of Patients with Tick-Borne Encephalitis (TBE): A European Multicentre Study from 2010 to 2017. Microorganisms. 2021;9(7):1420. doi:10.3390/microorganisms9071420
- Nygren TM, Pilic A, Böhmer MM, et al. Tick-borne encephalitis: Acute clinical manifestations and severity in 581 cases from Germany, 2018–2020. Journal of Infection. 2023;86(4):369-375. doi:10.1016/j.jinf.2023.02.018
- Nygren TM, Pilic A, Böhmer MM, Wagner-Wiening C, Wichmann O, Hellenbrand W. Recovery and sequelae in 523 adults and children with tick-borne encephalitis in Germany. Infection. 2023;51(5):1503-1511. doi:10.1007/s15010-023-02023-w
- Caracciolo I, Bassetti M, Paladini G, et al. Persistent viremia and urine shedding of tick-borne encephalitis virus in an infected immunosuppressed patient from a new epidemic cluster in North-Eastern Italy. J Clin Virol. 2015;69:48-51. doi:10.1016/j.jcv.2015.05.019
- de Bruijn M, van der Lely N, Marcelis J, Roks G. [’Tick-borne’ encephalitis in an immunocompromised patient]. Ned Tijdschr Geneeskd. 2015;159:A9067.
- Chmelík V, Chrdle A, Růžek D. Fatal tick-borne encephalitis in an immunosuppressed 12-year-old patient. Journal of Clinical Virology. 2016;74:73-74. doi:10.1016/j.jcv.2015.11.029
- Lipowski D, Popiel M, Perlejewski K, et al. A Cluster of Fatal Tick-borne Encephalitis Virus Infection in Organ Transplant Setting. The Journal of Infectious Diseases. 2017;215(6):896-901. doi:10.1093/infdis/jix040
- Askling HH, Insulander M, Hergens MP, Leval A. Tick borne encephalitis (TBE)-vaccination coverage and analysis of variables associated with vaccination, Sweden. Vaccine. 2015;33(38):4962-4968. doi:10.1016/j.vaccine.2015.07.030
- Riccò M, Corrado S, Marchesi F, Bottazzoli M. Tick-Borne Encephalitis Virus Vaccination among Tourists in a High-Prevalence Area (Italy, 2023): A Cross-Sectional Study. Trop Med Infect Dis. 2023;8(11):491. doi:10.3390/tropicalmed8110491
- Nygren TM, Pilic A, Böhmer MM, et al. Tick-borne encephalitis vaccine effectiveness and barriers to vaccination in Germany. Sci Rep. 2022;12:11706. doi:10.1038/s41598-022-15447-5
- Stefanoff P, Rosinska M, Samuels S, White DJ, Morse DL, Randolph SE. A National Case-Control Study Identifies Human Socio-Economic Status and Activities as Risk Factors for Tick-Borne Encephalitis in Poland. PLoS One. 2012;7(9):e45511. doi:10.1371/journal.pone.0045511
- Caputo M, Stumpe V, Rübsamen N, Mikolajczyk RT, Karch A. Implementation of preventive measures against tick-borne infections in a non-endemic area for tick-borne encephalitis—Results from a population-based survey in Lower Saxony, Germany. Ticks and Tick-borne Diseases. 2019;10(3):614-620. doi:10.1016/j.ttbdis.2019.02.005
- Kubinski M, Beicht J, Gerlach T, Volz A, Sutter G, Rimmelzwaan GF. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines (Basel). 2020;8(3). doi:10.3390/vaccines8030451
- Barrett PN, Schober-Bendixen S, Ehrlich HJ. History of TBE vaccines. Vaccine. 2003;21 Suppl 1:S41-9. doi:10.1016/s0264-410x(02)00814-9
- Lu Z, Broker M, Liang G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. 2008;8(5):713-720. doi:10.1089/vbz.2008.0028
- Xing Y, Schmitt HJ, Arguedas A, Yang J. Tick-borne encephalitis in China: A review of epidemiology and vaccines. Vaccine. 2017;35(9):1227-1237. doi:10.1016/j.vaccine.2017.01.015
- Federal Office for Public Health Switzerland. Federal Commission for Vaccination Issues, Swiss Vaccination Plan 2023. Bundesamt für Gesundheit, Direktionsbereich Prävention und Gesundheitsversorgung, Abteilung Übertragbare Krankheiten; 2023:46. Accessed December 18, 2023. https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.html
- Finnisch Institute for Health and Welfare. Finnish National Vaccination Programme.; 2023. Accessed December 29, 2023. https://thl.fi/en/web/infectious-diseases-and-vaccinations/information-about-vaccinations/finnish-national-vaccination-programme
- Federal Public Service Health, Food Chain Safety and Environment, Belgium. Superior Health Council. Vaccination against Tick-Borne Encephalitis (TBE). Brussels: SHC; 2019. Report 9435.; 2019. Accessed December 29, 2023. www.css-hgr.be
- Sākumlapa | Slimību profilakses un kontroles centrs. Accessed March 10, 2024. https://www.spkc.gov.lv/lv?
- Latvia extends encephalitis vaccine interval – Global Health Press. Accessed March 10, 2024. https://id-ea.org/latvia-extends-encephalitis-vaccine-interval/
- Schelling J, Einmahl S, Torgler R, Larsen CS. Evidence for a 10-year TBE vaccine booster interval: an evaluation of current data. Expert Review of Vaccines. 2024;23(1):226-236. doi:10.1080/14760584.2024.2311359
- Kind A, Ritzmann P, Marty F, Zimmermann H. Der Impfschutz gegen die Zeckenenzephalitis hält viel länger als bisher angenommen. Tick-borne encephalitis (TBE) – antibody titers and long-term immunity. Z Allg Med. 2008;84:153-156.
- Steffen R, Erber W, Schmitt HJ. Can the booster interval for the tick-borne encephalitis (TBE) vaccine “FSME-IMMUN” be prolonged? – A systematic review. Ticks Tick Borne Dis. 2021;12(5):101779. doi:10.1016/j.ttbdis.2021.101779
- Federal Office for Public Health Switzerland. Cantonal Vaccination Monitoring.; 2022. Accessed February 6, 2024. https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/informationen-fachleute-gesundheitspersonal/durchimpfung.html
- Schmidt AJ, Altpeter E, Graf S, Steffen R. Tick-borne encephalitis (TBE) in Switzerland: does the prolongation of vaccine booster intervals result in an increased risk of breakthroughs? J Travel Med. 2022;29(2). doi:10.1093/jtm/taab158
- Angulo FJ, Zhang P, Halsby K, et al. A systematic literature review of the effectiveness of tick-borne encephalitis vaccines in Europe. Vaccine. 2023;41(47):6914-6921. doi:10.1016/j.vaccine.2023.10.014
- Kunz C. TBE vaccination and the Austrian experience. Vaccine. 2003;21 Suppl 1:S50-5. doi:10.1016/s0264-410x(02)00813-7
- Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25(43):7559-7567. doi:10.1016/j.vaccine.2007.08.024
- Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19(1):69-76. doi:10.3201/eid1901.120458
- Chitimia-Dobler L, Lindau A, Oehme R, et al. Tick-Borne Encephalitis Vaccination Protects from Alimentary TBE Infection: Results from an Alimentary Outbreak. Microorganisms. 2021;9(5):889. doi:10.3390/microorganisms9050889
- Pugh SJ, Moïsi JC, Kundi M, et al. Effectiveness of two doses of tick-borne encephalitis (TBE) vaccine. J Travel Med. 2022;29(2). doi:10.1093/jtm/taab193
- Zens KD, Haile SR, Schmidt AJ, Altpeter ES, Fehr JS, Lang P. Retrospective, matched case-control analysis of tickborne encephalitis vaccine effectiveness by booster interval, Switzerland 2006-2020. BMJ Open. 2022;12(4):e061228. doi:10.1136/bmjopen-2022-061228
- Zavadska D, Freimane Z, Karelis G, et al. Effectiveness of tick-borne encephalitis vaccination in Latvia, 2018-2020: an observational study. Clin Microbiol Infect. Published online July 6, 2023. doi:10.1016/j.cmi.2023.06.028
- Zavadska D, Freimane Z, Karelis G, et al. Effectiveness of Tick-borne Encephalitis Vaccines in Children, Latvia, 2018-2020. Pediatr Infect Dis J. 2023;42(10):927-931. doi:10.1097/inf.0000000000004034
- Kynčl J, Angulo FJ, Orlikova H, et al. Effectiveness of vaccination against tick-borne encephalitis in the Czech Republic, 2018-2022: an observational study. Submitted. Published online 2023.
- Erber W, Schmitt HJ. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick Borne Dis. 2018;9(4):768-777. doi:10.1016/j.ttbdis.2018.02.007
- Zens Kyra D, Altpeter Ekkehardt, Wymann Monica N, Mack Annora, Baer Nora B, Haile Sarah R, Steffen Robert, Fehr Jan S, Lang Phung. A combined cross-sectional analysis and case-control study evaluating tick-borne encephalitis vaccination coverage, disease and vaccine effectiveness in children and adolescents, Switzerland, 2005 to 2022. Euro Surveill. 2024;29(18):pii=2300558. https://doi.org/10.2807/1560-7917.ES.2024.29.18.2300558
- Rampa JE, Askling HH, Lang P, et al. Immunogenicity and safety of the tick-borne encephalitis vaccination (2009–2019): A systematic review. Travel Medicine and Infectious Disease. 2020;37:101876. doi:10.1016/j.tmaid.2020.101876
- World Health Organization. Tick-borne encephalitis. Accessed March 11, 2024. https://www.who.int/health-topics/tick-borne-encephalitis
- Ehrlich HJ, Pavlova BG, Fritsch S, et al. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: evaluation of safety and immunogenicity. Vaccine. 2003;22(2):217-223. doi:10.1016/S0264-410X(03)00563-2
- Zent O, Banzhoff A, Hilbert AK, Meriste S, Słuzewski W, Wittermann C. Safety, immunogenicity and tolerability of a new pediatric tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. Vaccine. 2003;21(25):3584-3592. doi:10.1016/S0264-410X(03)00421-3
- Pöllabauer EM, Fritsch S, Pavlova BG, et al. Clinical evaluation to determine the appropriate paediatric formulation of a tick-borne encephalitis vaccine. Vaccine. 2010;28(29):4558-4565. doi:10.1016/j.vaccine.2010.04.075
- Wittermann C, Izu A, Petri E, Gniel D, Fragapane E. Five year follow-up after primary vaccination against tick-borne encephalitis in children. Vaccine. 2015;33(15):1824-1829. doi:10.1016/j.vaccine.2015.02.038
- Pöllabauer EM, Pavlova BG, Löw-Baselli A, et al. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine. 2010;28(29):4680-4685. doi:10.1016/j.vaccine.2010.04.047
- Kaiser R. Frühsommermeningoenzephalitis im Kindes- und Jugendalter. Monatsschr Kinderheilkd. 2006;154(11):1111-1116. doi:10.1007/s00112-005-1184-4
- Bogdanavičienė K, Gudavičiūtė G, Šeškutė M. A Retrospective Analysis of Tick-borne Encephalitis in Children Treated in Kaunas Hospital During 2012 to 2019. The Pediatric Infectious Disease Journal. 2022;41(9):702. doi:10.1097/INF.0000000000003595
- Lesnicar G, Poljak M, Seme K, Lesnicar J. Pediatric tick-borne encephalitis in 371 cases from an endemic region in Slovenia, 1959 to 2000. Pediatr Infect Dis J. 2003;22(7):612-617. doi:10.1097/01.inf.0000073202.39700.a0
- Krbková L, Štroblová H, Bednářová J. Clinical course and sequelae for tick-borne encephalitis among children in South Moravia (Czech Republic). Eur J Pediatr. 2015;174(4):449-458. doi:10.1007/s00431-014-2401-8
- Bojkiewicz E, Toczylowski K, Grygorczuk S, et al. The Prevalence of Asymptomatic Infections with Tick-Borne Encephalitis Virus and Attitude towards Tick-Borne Encephalitis Vaccine in the Endemic Area of Northeastern Poland. Vaccines. 2022;10(8):1294. doi:10.3390/vaccines10081294
- Sundin M. Chapter 6: TBE in children. TBE Book. Published December 28, 2016. Accessed February 13, 2024. https://tbenews.com/tbe/tbe6/
- Fritsch P, Gruber-Sedlmayr U, Pansi H, et al. Tick-borne encephalitis in Styrian children from 1981 to 2005: a retrospective study and a review of the literature. Acta Paediatrica. 2008;97(5):535-538. doi:10.1111/j.1651-2227.2008.00763.x
- Parfut A, Laugel E, Baer S, et al. Tick-borne encephalitis in pediatrics: An often overlooked diagnosis. Infectious Diseases Now. 2023;53(2):104645. doi:10.1016/j.idnow.2023.01.005
- Stähelin-Massik J, Zimmermann H, Gnehm HE. Tick-Borne Encephalitis in Swiss Children 2000–2004: Five-Year Nationwide Surveillance of Epidemiologic Characteristics and Clinical Course. The Pediatric Infectious Disease Journal. 2008;27(6):555. doi:10.1097/INF.0b013e318165c195
- Iff T, Meier R, Olah E, Schneider JFL, Tibussek D, Berger C. Tick-borne meningo-encephalitis in a 6-week-old infant. Eur J Pediatr. 2005;164(12):787-788. doi:10.1007/s00431-005-1753-5
- Schmolck H, Maritz E, Kletzin I, Korinthenberg R. Neurologic, Neuropsychologic, and Electroencephalographic Findings After European Tick-borne Encephalitis in Children. J Child Neurol. 2005;20(6):500-508. doi:10.1177/088307380502000606
- Zenz W, Pansi H, Zoehrer B, et al. Tick-Borne Encephalitis in Children in Styria and Slovenia Between 1980 and 2003. The Pediatric Infectious Disease Journal. 2005;24(10):892. doi:10.1097/01.inf.0000180506.76201.43
- Lindquist L, Vapalahti O. Tick-borne encephalitis. The Lancet. 2008;371(9627):1861-1871. doi:10.1016/S0140-6736(08)60800-4
- Haglund M, Günther G. Tick-borne encephalitis—pathogenesis, clinical course and long-term follow-up. Vaccine. 2003;21:S11-S18. doi:10.1016/S0264-410X(02)00811-3
- Karelis G, Bormane A, Logina I, et al. Tick-borne encephalitis in Latvia 1973–2009: epidemiology, clinical features and sequelae. European Journal of Neurology. 2012;19(1):62-68. doi:10.1111/j.1468-1331.2011.03434.x
- Mickienė A, Laiškonis A, Günther G, Vene S, Lundkvist Å, Lindquist L. Tickborne Encephalitis in an Area of High Endemicity in Lithuania: Disease Severity and Long-Term Prognosis. Clinical Infectious Diseases. 2002;35(6):650-658. doi:10.1086/342059
- Veje M, Nolskog P, Petzold M, et al. Tick-Borne Encephalitis sequelae at long-term follow-up: a self-reported case–control study. Acta Neurologica Scandinavica. 2016;134(6):434-441. doi:10.1111/ane.12561
- Bogovič P, Stupica D, Rojko T, et al. The long-term outcome of tick-borne encephalitis in Central Europe. Ticks and Tick-borne Diseases. 2018;9(2):369-378. doi:10.1016/j.ttbdis.2017.12.001
- Fowler Å, Forsman L, Eriksson M, Wickström R. Tick-Borne Encephalitis Carries a High Risk of Incomplete Recovery in Children. The Journal of Pediatrics. 2013;163(2):555-560. doi:10.1016/j.jpeds.2013.01.037
- Engman ML, Lindström K, Sallamba M, et al. One-year Follow-up of Tick-borne Central Nervous System Infections in Childhood. The Pediatric Infectious Disease Journal. 2012;31(6):570. doi:10.1097/INF.0b013e31824f23c0
- Henrik U, Åsa F, Ronny W. Increased working memory related fMRI signal in children following Tick Borne Encephalitis. European Journal of Paediatric Neurology. 2016;20(1):125-130. doi:10.1016/j.ejpn.2015.09.004
- UN Tourism Dashboard. Global and regional tourism performance. Available online: Accessed January 30, 2024. https://www.unwto.org/tourism-data/global-and-regional-tourism-performance
- Banzhoff A, Bröker M, Zent O. Protection against tick-borne encephalitis (TBE) for people living in and travelling to TBE-endemic areas. Travel Medicine and Infectious Disease. 2008;6(6):331-341. doi:10.1016/j.tmaid.2008.06.011
- Steffen R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. Journal of Travel Medicine. 2016;23(4):taw018. doi:10.1093/jtm/taw018
- Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus – a review of an emerging zoonosis. Journal of General Virology. 2009;90(8):1781-1794. doi:10.1099/vir.0.011437-0
- Chrdle A, Chmelík V, Růžek D. Tick-borne encephalitis: What travelers should know when visiting an endemic country. Hum Vaccin Immunother. 2016;12(10):2694-2699. doi:10.1080/21645515.2016.1218098
- World Health Organization. Position paper on Tick-borne Encephalitis 2011. Accessed March 11, 2024. https://iris.who.int/bitstream/handle/10665/241769/WER8624_241-256.PDF?sequence=1
- Federal Office for Public Health Switzerland. Zeckenübertragene Krankheiten – Lagebericht Schweiz. Accessed March 12, 2024. https://www.bag.admin.ch/bag/de/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/zeckenuebertragene-krankheiten.html
- Briggs BJ, Atkinson B, Czechowski DM, et al. Tick-Borne Encephalitis Virus, Kyrgyzstan. Emerg Infect Dis. 2011;17(5):876-879. doi:10.3201/eid1705.101183
- Marano C, Moodley M, Melander E, De Moerlooze L, Nothdurft HD. Perceptions of tick-borne encephalitis risk: a survey of travellers and travel clinics from Canada, Germany, Sweden and the UK. Journal of Travel Medicine. 2019;26(Supplement_1):S10-S16. doi:10.1093/jtm/tay063
- Poulos C, Boeri M, Coulter J, Huang L, Schley K, Pugh SJ. Travelers’ preferences for tick-borne encephalitis vaccination. Expert Review of Vaccines. 2022;21(10):1495-1504. doi:10.1080/14760584.2022.2108798
- Hills SL, Broussard KR, Broyhill JC, et al. Tick-borne encephalitis among US travellers, 2010–20. Journal of Travel Medicine. 2022;29(2):taab167. doi:10.1093/jtm/taab167
- Park M, Jit M, Wu JT. Cost-benefit analysis of vaccination: a comparative analysis of eight approaches for valuing changes to mortality and morbidity risks. BMC Medicine. 2018;16(1):139. doi:10.1186/s12916-018-1130-7
- Schwarz B. [Health economics of early summer meningoencephalitis in Austria. Effects of a vaccination campaign 1981 to 1990]. Wien Med Wochenschr. 1993;143(21):551-555.
- Smit R. Cost-effectiveness of tick-borne encephalitis vaccination in Slovenian adults. Vaccine. 2012;30(44):6301-6306. doi:10.1016/j.vaccine.2012.07.083
- Haglund M, Forsgren M, Lindh G, Lindquist L. A 10-year follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand J Infect Dis. 1996;28(3):217-224. doi:10.3109/00365549609027160
- Cassini A, Colzani E, Pini A, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23(16). doi:10.2807/1560-7917.Es.2018.23.16.17-00454
- Fafangel M, Cassini A, Colzani E, et al. Estimating the annual burden of tick-borne encephalitis to inform vaccination policy, Slovenia, 2009 to 2013. Euro Surveill. 2017;22(16). doi:10.2807/1560-7917.Es.2017.22.16.30509
- Cassini A, Colzani E, Kramarz P, Kretzschmar ME, Takkinen J. Impact of food and water-borne diseases on European population health. Current Opinion in Food Science. 2016;12:21-29. doi:10.1016/j.cofs.2016.06.002
- Shedrawy J, Henriksson M, Hergens MP, Askling HH. Estimating costs and health outcomes of publicly funded tick-born encephalitis vaccination: A cost-effectiveness analysis. Vaccine. 2018;36(50):7659-7665. doi:10.1016/j.vaccine.2018.10.086
- Cizman M, Rakar R, Zakotnik B, Pokorn M, Arnez M. Severe forms of tick-borne encephalitis in children. Wien Klin Wochenschr. 1999;111(12):484-487.
- Prata JC, Ribeiro AI, Rocha-Santos T. Chapter 1 – An introduction to the concept of One Health. In: Prata JC, Ribeiro AI, Rocha-Santos T, eds. One Health. Academic Press; 2022:1-31. doi:10.1016/B978-0-12-822794-7.00004-6
- Süss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine. 2003;21:S19-S35. doi:10.1016/S0264-410X(02)00812-5
- Michelitsch A, Wernike K, Klaus C, Dobler G, Beer M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses. 2019;11(7):669. doi:10.3390/v11070669
- Gonzalez G, Bournez L, Moraes RA, et al. A One-Health Approach to Investigating an Outbreak of Alimentary Tick-Borne Encephalitis in a Non-endemic Area in France (Ain, Eastern France): A Longitudinal Serological Study in Livestock, Detection in Ticks, and the First Tick-Borne Encephalitis Virus Isolation and Molecular Characterisation. Front Microbiol. 2022;13:863725. doi:10.3389/fmicb.2022.863725
- Süss J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks and Tick-borne Diseases. 2011;2(1):2-15. doi:10.1016/j.ttbdis.2010.10.007
- Hofmeester TR, Sprong H, Jansen PA, Prins HHT, van Wieren SE. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit Vectors. 2017;10:433. doi:10.1186/s13071-017-2370-7
- Jaenson TGT, Petersson EH, Jaenson DGE, et al. The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: incidence of human TBE correlates with abundance of deer and hares. Parasites & Vectors. 2018;11(1):477. doi:10.1186/s13071-018-3057-4
- Kjær LJ, Johansson M, Lindgren PE, et al. Potential drivers of human tick-borne encephalitis in the Örebro region of Sweden, 2010–2021. Sci Rep. 2023;13(1):7685. doi:10.1038/s41598-023-34675-x
- Rubel F, Brugger K. Operational TBE incidence forecasts for Austria, Germany, and Switzerland 2019-2021. Ticks Tick Borne Dis. 2021;12(1):101579. doi:10.1016/j.ttbdis.2020.101579
- Kríz B, Benes C, Daniel M. Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997-2008). Epidemiol Mikrobiol Imunol. 2009;58(2):98-103.
- Kerlik J, Avdičová M, Štefkovičová M, et al. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: Analysis of tick-borne encephalitis outbreaks in Slovakia during 2007–2016. Travel Medicine and Infectious Disease. 2018;26:37-42. doi:10.1016/j.tmaid.2018.07.001
- French Agency for Food, Environmental and Occupational Health & Safety. Tick-borne encephalitis : tracing the origin of cases of transmission via cheese. Anses – Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. Published October 4, 2022. Accessed March 11, 2024. https://www.anses.fr/en/content/tick-borne-encephalitis-transmission-cheese
- 21bites. Embracing the Future of Food: Top Food Trends to Watch in 2024. 21bites. Accessed March 7, 2024. https://21bites.com/blogs/blog/embracing-the-future-of-food-top-food-trends-to-watch-in-2024
- Slunge D. The Willingness to Pay for Vaccination against Tick-Borne Encephalitis and Implications for Public Health Policy: Evidence from Sweden. PLoS One. 2015;10(12):e0143875. doi:10.1371/journal.pone.0143875
- Kollaritsch H, Chmelík V, Dontsenko I, et al. The current perspective on tick-borne encephalitis awareness and prevention in six Central and Eastern European countries: Report from a meeting of experts convened to discuss TBE in their region. Vaccine. 2011;29(28):4556-4564. doi:10.1016/j.vaccine.2011.04.061