Background
Tick-borne encephalitis (TBE) is a severe neurologic disease in Europe and Asia with disease expression ranging from asymptomatic to severe neurological clinical pictures and potentially fatal outcome. While central nervous system (CNS) symptomatology is mostly described in TBEV infection, gastrointestinal (GI) symptoms in humans are sporadically reported. Kaiser et al.1 conducted a prospective study including 656 German TBE patients between 1994 and 1998 where an impaired bowel function was reported in 1% of patients with meningoencephalitis and 13.5% of patients with meningoencephalomyelitis. In addition, Kleiter et al.2 reported three TBE patients displaying autonomic dysfunction in the upper and/or lower GI tract. Symptoms included severe postprandial abdominal pain and vomiting, reduced bowel motility and constipation. Investigations on TBEV-infection associated GI symptoms have been made int the mouse model. Nagata et al.3 observed infection of enteric neurons in intravenously infected mice displaying intestinal distension caused by peripheral neuritis and paresis. White et al.4 reported similar observations in C57BL/6 mice after subcutaneous flaviviral (West Nile virus, Zika virus and Powassan virus) infection. Flaviviral infection resulted in delayed GI transit and a GI tract dysmotility abnormalities due to infection and damage to enteric neurons.Within this study, the occurrence of GI symptoms in a more natural infection model was analyzed by infecting immunocompetent C57BL/6JOlaHsd mice subcutaneously with 1.000 viral particles of the European TBEV prototype strain Neudoerfl. Both, mice and virus strain, were chosen because they constitute well characterized models and to increase the comparability of this study with other studies on flavivirus infections in model systems.4
Results
Within this study, 10 of 22 subcutaneously infected mice displayed acute distension and segmental dilation of the intestinal tract in addition to other symptoms like bodyweight loss, ruffed fur, hunchback posture, hind leg paresis and moribund condition. Those macroscopic alterations of the GI tract first occurred at 8 days post infection (dpi) including considerable segmental dilation and distension of the stomach and the small intestine (Figure 1B,D) compared to mock-infected mice (Figure 1A,C). The proximal and mid part of the small intestine were most affected (Table 1). In contrast, none of the intracerebral infected positive as well as mock-infected controls displayed evidence of bowel dilation. Hematoxylin and eosin staining revealed an intramural ganglioneuritis with infiltrating inflammatory cells of the myenteric and submucosal plexus within small and/or large intestine. An enteric ganglioneuritis was observed in 15 subcutaneously infected mice as well as both positive control mice (Table 1). Ganglioneuritis was detected in one mouse at day 2 and day 4, in two mice at day 7 and subsequently, in all further TBEV-infected mice (11 mice) with varying severity (Table 1). Staining with an anti-TBEV-rabbit serum revealed intracytoplasmic viral antigen in the enteric plexus of eight subcutaneously TBEV-infected mice from day 7 onwards as well as in one positive control mouse at day 6. Furthermore, significant higher numbers of infiltrating CD3+ T cells and CD107b+ macrophages were observed in symptomatic animals. CD3+ T cells were the most abundant inflammatory cell type found within the GI tract and highest numbers correlated with presence of ganglioneuritis as well as with macroscopic GI tract alterations.
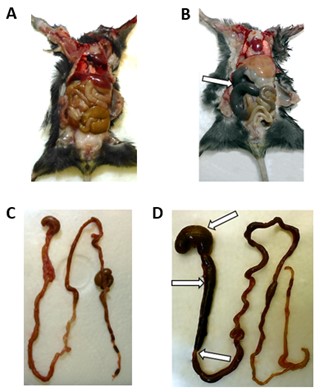
Figure 1: Macroscopic findings of the GI tract from subcutaneously TBEV-infected mice ((B) M26 at 8 dpi; (D) M22 at 10 dpi) and mock-infected control M1 at 14 dpi (A,C). Segmental distension and alterations of the small intestine and the stomach are indicated by white arrows. Acute GI tract alterations were found in subcutaneously TBEV-infected mice after 8 dpi.5
Table 1: Macroscopic Overview of mice with enteral ganglioneuritis (15 of 22) after subcutaneous TBEV infection with the day of euthanasia after infection and clinical score (HEP= humane endpoint). The severity degree of ganglioneuritis ((+) = minimal, + = mild, ++ = mild to moderate, +++ = moderate, – = absent), presence of viral antigen (+ =present, – = absent), presence of macroscopic alterations in GI tract regions (+ = present, – = absent) and status of viral RNA copies presence in intestine measured by qRT-PCR (+ =present, – = absent) are given for each animal. Neudoerfl strain. M29 and M30 were intracerebrally infected positive control mice. No enteral ganglioneuritis, TBEV antigen, RNA or macroscopical alterations were found in the mock-infected negative control mice (data not shown).5
DiscussionThis study demonstrates the presence of acute GI alterations after subcutaneous TBEV infection with the European prototype TBEV strain Neudoerfl in C57BL/6JOlaHsd mice caused by ganglioneuritis in the myenteric and submucosal plexus of small and large intestine. GI tract alterations presented as abdominal distension and segmental dilation of the small intestine and stomach acutely appearing in all animals except one mouse after 8 dpi. In animals presenting with macroscopic alterations of the GI tract and ganglioneuritis significant high amounts of infiltrating CD3+ T lymphocytes and, to a lesser amount, macrophages were observed. These findings are in line with observations for intravenously TBEV-infected mice3 as well as for mice subcutaneously challenged with different neurotropic flaviviruses.4 Therefore, infection and damage (virus- and/or immune-mediated) of neurons of the enteric nervous system by neurotropic flaviviruses might be source of acute and persistent GI pathology and dysmotility. Regarding this, the impact in life quality resulting from flavivirus-induced GI complications and sequelae should not be neglected. Additionally, further investigations on TBE-induced pathogenesis to the GI tract for the tick-borne as well as the food-borne infection routes are required.
Literature- Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994-98: A prospective study of 656 patients. Brain. 1999;122 (Pt 11):2067–2078.
- Kleiter I, Steinbrecher A, Flugel D, Bogdahn U, Schulte-Mattler W. Autonomic involvement in tick-borne encephalitis (TBE): Report of five cases. Eur J Med Res. 2006;11(6):261–265.
- Nagata N, Iwata-Yoshikawa N, Hayasaka D, Sato Y, Kojima A, Kariwa H, Takashima I, Takasaki T, Kurane I, Sata T, et al. The pathogenesis of 3 neurotropic flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J Neuropathol Exp Neurol. 2015;74(3):250–260.
- White JP, Xiong S, Malvin NP, Khoury-Hanold W, Heuckeroth RO, Stappenbeck TS, Diamond MS. Intestinal Dysmotility Syndromes following Systemic Infection by Flaviviruses. Cell. 2018;175(5):1198–1212.
- Boelke M, Puff C, Becker K, Hellhammer F, Gusmag F, Marks H, Liebig K, Stiasny K, Dobler G, Baumgärtner W, Schulz C, Becker SC, Enteric Ganglioneuritis, a Common Feature in a Subcutaneous TBEV Murine Infection Model. Microorganisms. 2021;9(4):875.
Author: Dr. Mathias Boelke
Dr. rer. nat. Mathias Boelke is a biologist currently working in the working group of Prof. Dr. rer. nat. Stefanie Becker for the Institute for Parasitology and Research Center for Emerging Infections and Zoonoses (RIZ), University of Veterinary Medicine Hannover, Germany. The above research was conducted as part of his PhD thesis titled, “Characterization of tick-borne encephalitis virus in questing ticks and in a murine infection model”, and from identification and phylogenetic analysis of TBEV in the German non-endemic federal state of Lower Saxony. He is currently conducting further research on TBEV-tick infection studies as well as identification and characterization of tick-borne viruses.
Compiled: July 2021