Background
All arthropod-borne viruses depend on their respective vectors for persistence in nature and transmission to susceptible hosts. Thus, it is logical that endemic areas for the virus and the vector mostly overlap, as is the case for almost all mosquito-transmitted diseases such as Dengue or yellow fever [1]. The tick transmitted tick-borne encephalitis virus (TBEV), which is mainly vectored by Ixodes ricinus ticks in Europe, seems to be an exception from this rule. Although the vector (Ixodes ricinus), as well as the preferred host (Myodes glareolus), is widely distributed in Europe, and climatic conditions and habitat parameters are suitable for TBEV transmission in many locations, the occurrence of TBEV transmission is focal and often restricted to a small parcel of land. Thus, we need to ask the question of which factors shape this focal distribution of TBEV in the natural habitat?Driving factors for TBEV transmission might be too numerous and diffuse to answer this question with a single study. However, we can start step by step to confirm or discard hypotheses about potential drivers for TBEV emergence. With the current study, we tested the hypothesis that tick population and virus isolate have an evolutionary connection with each other and by co-developing have adapted to each other, which might help to explain the remarkable stability of TBEV isolates in the natural TBEV foci.
Results & discussion
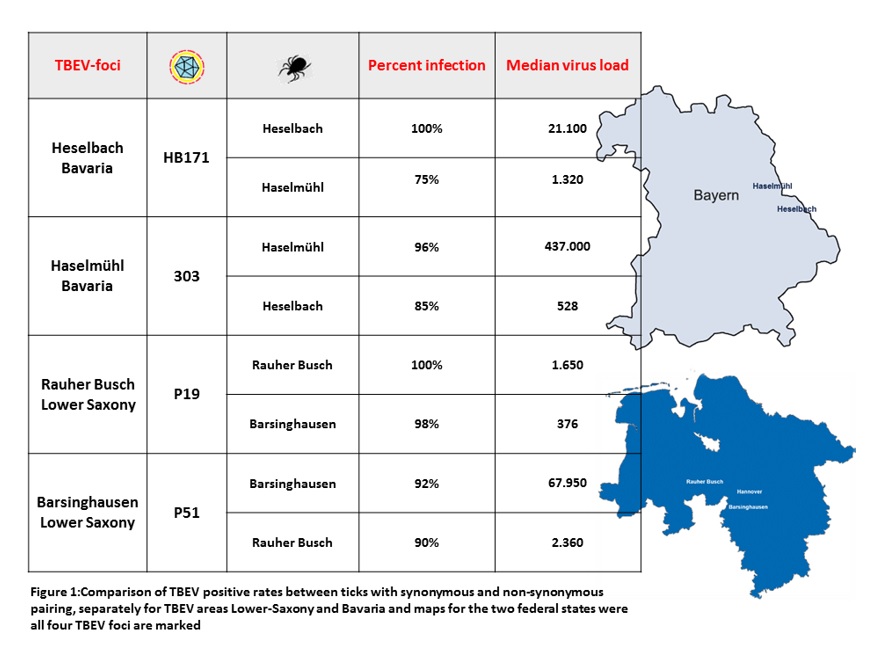
To analyze the role of tick and virus genotypes, we made use of an artificial blood-feeding system to induce TBEV infection in field-collected nymphal ticks. Through studies by us [2] and Gerhard Dobler and colleagues [3], we had information on ticks and virus isolates for two natural TBEV foci in Lower Saxony and two foci in Bavaria which could be used as a showcase for our analysis (Figure 1). Both pairs, Lower Saxony (Rauher Busch and Barsinghausen-Mooshütte) and Bavaria (Haselmühl and Heselbach) were located in close proximity with only around 40 km distance (beeline) and TBEV isolates were genetically closely related but had 11 and 19 amino acids difference. These facts made them an ideal test set for our hypothesis. The ticks from the foci Barsinghausen-Mooshütte and Rauher Busch were fed either with blood containing TBEV isolate P51 (Barsinghausen) or P19 (Rauher Busch), and ticks from the TBEV-foci Haselmühl and Heselbach were fed with blood containing either TBEV isolate 303/16 (Haselmühl) or HB171 (Heselbach). The infection of a tick with the isolate from the same focus was called synonymous infection whereas the infection with the closely related virus from the neighboring focus was called non-synonymous infection [4].
After blood feeding, we incubated the ticks for 7 days and measured the infection success of TBEV by qRT-PCR [5]. This allowed us to analyze the infection rate and the viral replication rates illustrated by viral RNA copies per tick (Figure 1). We found that infection rates were extraordinarily high in 2020 as compared to previous years (mean 38%, [6]). The Infection rates with the non-synonymous infection were always slightly lower than with the synonymous virus. To test if the higher infection rate is statistically significant, we used Fisher’s exact test and odds ratios. Only for the focus Heselbach a significant correlation between synonymous infection and infection rates was found (p=0.0026) which then contributed to the overall significance observed for the state Bavaria (Odds ratio for synonymous infection 14.5, p=0.0014).
More obviously, median viral RNA copy numbers were significantly higher in the synonymous virus-tick population pairings. This was true for all four populations tested (Figure 1). However, since we used field-collected ticks from natural foci, they might already carry an infection before we feed them, and the blood meal might even increase those viral titers [7]. Thus, to study the robustness of our results, we removed outliers, which were defined as exceptionally high viral loads. Using the boxplot method, we removed 12, 17, 4, and 17 outliers from the data for Barsinghausen-Mooshütte, Haselmühl, Heselbach and Rauher Busch, respectively. With this extreme process of outlier removal, the data for Heselbach remain significant and Rauher Busch (p=0.0525) and Haselmühl (p=0.07) remain slightly above the significance level. This led us to the conclusion, that even if some pre-infected ticks would have been used in our study, the results are not corrupted and our conclusion stays the same.
Conclusion
In conclusion, our study provides the first evidence for a virus isolate-tick population relationship that could be responsible for the focal distribution of TBEV transmission. Which genetic factors in ticks and viruses shape this relationship remains to be further investigated.
Literature
1. Kamal M, Kenawy MA, Rady MH, Khaled AS, Samee AM (2018) Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 13(12): e0210122. https://doi.org/10.1371/journal.pone.0210122
2. Boelke M, Bestehorn M, Marchwald B, Kubinski M, Liebig K, Glanz J, Schulz C, Dobler G, Monazahian M, Becker SC. First Isolation and Phylogenetic Analyses of Tick-Borne Encephalitis Virus in Lower Saxony, Germany. Viruses. 2019 May 21;11(5):462. doi: 10.3390/v11050462. PMID: 31117224
3. Dobler G, Bestehorn M, Antwerpen M, Överby-Wernstedt A. Complete Genome Sequence of a Low-Virulence Tick-Borne Encephalitis Virus Strain. Genome Announc. 2016;4(5):e01145-16. Published 2016 Oct 20. doi:10.1128/genomeA.01145-16
4. Liebig K, Boelke M, Grund D, Schicht S, Bestehorn-Willmann M, Chitimia-Dobler L, Dobler G, Jung K, Becker SC. The Stable Matching Problem in TBEV Enzootic Circulation: How Important Is the Perfect Tick-Virus Match? Microorganisms. 2021 Jan 19;9(1):196. doi: 10.3390/microorganisms9010196.
5. Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick-borne encephalitis virus (TBEV) RNA. Journal of Clinical Virology: the Official Publication of the Pan American Society for Clinical Virology. 2003 Jul;27(2):136-145. DOI: 10.1016/s1386-6532(02)00168-3.
6. Liebig K, Boelke M, Grund D, Schicht S, Springer A, Strube C, Chitimia-Dobler L, Dobler G, Jung K, Becker S. Tick populations from endemic and non-endemic areas in Germany show differential susceptibility to TBEV. Sci Rep. 2020 Sep 23;10(1):15478. doi: 10.1038/s41598-020-71920-z.
7. Belova OA, Burenkova LA, Karganova GG. Different tick-borne encephalitis virus (TBEV) prevalences in unfed versus partially engorged ixodid ticks–evidence of virus replication and changes in tick behavior. Ticks Tick Borne Dis. 2012 Sep;3(4):240-6. doi: 10.1016/j.ttbdis.2012.05.005.
Author: Prof. Dr. Stefanie Becker
Prof. Dr. rer. nat. Stefanie Becker is a Professor at the Institute for Parasitology and group leader at the Research Center for Emerging Infections and Zoonoses, University of Veterinary Medicine Hannover, Germany. She has worked on antiviral immunity in insects including the description of new antiviral molecules in insects and the role of RNA interference and induced immune pathways against a variety of arboviruses. Furthermore, she and her team studied vector abundance, genetic composition and vector competence of German mosquitos. Recently she has started several research projects on tick-borne viruses with special emphasis on vector competence and antiviral immunity of German Ixodes ricinus populations. She also worked on viral adaptation to mosquito and tick vectors and the discovery of novel tick-associated viruses in German tick populations.
Compiled: March 2021