Michael Kunze, Wilhelm Erber and Martin Haditsch
Key points
- The incidence of TBE ranges from ‘only single sporadic cases’ to >50/105 per year depending on the region and on the year of analysis; it is usually 1-10/105 in endemic regions in central Europe.
- This number may be considered as ‘low’ – not only as an individual risk but also from a public health perspective.
- If an individual does contract TBE, however, the disease may deeply change her/his life due to the need for acute hospital care and due to potentially severe and long-term sequelae. In 1–2% (-20%) of cases, TBE may even result in death.
- No specific treatments exist for TBE. The severity of the disease and high frequency of long-term sequelae result in high public awareness and concerns about tick bites in endemic areas. Public health officials in TBE-endemic areas need to address these concerns; moreover, they need to address the concerns of travelers at risk.
- The principal public health measures aim at reducing TBE cases by reduction of exposure and preventive vaccination.
- Recommendation/reimbursement of TBE vaccination still is under discussion from side of healthcare payer perspective as well as from the individuals perspective considering long term sequelae.
Introduction
Descriptive epidemiology is the cornerstone of information for public health considerations. In this regard, as outlined in various chapters of this book, tick-borne encephalitis (TBE) poses specific challenges:
- TBE presents as a non-specific CNS disease to family physicians, general practitioners, pediatricians, internal medicine specialists, neurologists, and other medical specialists. Especially outside of endemic areas, TBE is often not diagnosed because physicians are not aware of the differential diagnosis, and they do not order the appropriate test to confirm TBE infection. This phenomenon is particularly important in countries or regions where the burden of disease of TBE and perhaps even the presence of the TBE virus (TBEV) have not been fully studied. In some countries, even the costs and limited availability of serological testing for TBE serve as barriers to reaching a correct diagnosis.
- In many countries, incomplete reporting of TBE is likely. This fact starts a vicious cycle in which low TBE incidences or even periodic lack of human TBE cases result in low awareness and further underdiagnosis of the disease. As long as the risk is low (by regional / national definition) some ‘official maps’ published by governmental bodies do not indicate this risk for TBE in a specific region because a special ‘incidence threshold’ is used as a condition before TBE risk is communicated. Thus, lack of case finding and case reporting results in missed opportunities for prevention.
- With vaccine uptake being unknown in many instances, reporting case incidences results in artificially low numbers and thus an underestimation of true TBE risk.
- The risk of TBEV infection is influenced by seasonal patterns of tick bites and transmission, the environment, personal behaviour, personal protection measures and, of course, (vaccination-induced or natural) immunity. The details of the interactions among all parameters (reservoir animals, tick activity, migrating birds, climate, the environment/landscape and its changes over time, human behaviour, etc.) and the resulting risk for TBE is largely unknown to date and – due to the complexity – difficult to assess.
- The risk of TBE disease in general and the severity of specific symptoms depend on age, immunological status, underlying diseases, routine medications, TBEV viral load, and the specific infecting TBE strain. Not only has the epidemiology of TBE posed issues for public health officials, but it also appears fair to state that perhaps with the exception of Austria, TBE is 1) largely underreported and 2) mostly neglected by public health authorities. Several reasons may explain this phenomenon:
- TBE vaccination results in protection of the individual only. There is no herd protection because the viral reservoir exists outside the human population and – with the exception of an extremely low risk of transmission via breast-feeding or blood transfusion – TBE is not transmitted between humans.
- Classical TBE infection (i.e., infection involving the CNS) is relatively rare, so cost-benefit analyses are likely negative, particularly if long-term sequelae and social costs are not accounted for. The prerequisite for a vaccination program to be effective is a high vaccine uptake and this requires appropriate funding not only for the vaccine but also for its administration. The results then of such a program are ‘no disease’ – and absence of diseases is not a success story in the popular media or in elections unless rigorous (and again expensive) surveillance is undertaken to assess field effectiveness and document the success. Valid surveillance again needs appropriate funding – and so another vicious cycle emerges, where a perceived ‘rare disease’ is not considered to justify or even be eligible for public health expenditures.
- Public health officials often become more active largely when there are common threats, and symptomatic TBE is relatively rare (see Chapter 11b). Moreover, TBE vaccination results in individual protection only and not in any herd protection .(As indicated above apart from breast-feeding and blood transfusions in very rare instances TBEV has not been proven to be transmitted between humans). So, while TBE vaccination is highly effective, it does not result in any impact on the population in general; thus, TBE vaccination is often not paid for by public health resources.
- In some instances local governments prioritize tourism / travel over public health concerns and may be not in favour of indicating their region being classified as a TBE-risk-area.
We strongly believe – against all these arguments and despite the perceived low incidence of TBE – that this disease deserves a high level of public health attention because it poses a risk to any human living in or traveling to or through TBE endemic areas and because the disease may frequently result in long-term disability and, in some cases, even death. Rightfully so, the public should be concerned and, if correctly informed, would certainly opt for an adequate public health response.
As a response to all these public health challenges, and to encourage the control of TBE in Eurasia, an international effort was launched in 1999 with the aim to investigate and alleviate this situation. International experts created a new body, the International Scientific Working Group on Tick-Borne Encephalitis (ISW-TBE; www.iswtbe.com). This Working Group gathers data from internationally recognized scientific experts from TBEV endemic and non-endemic regions with extensive personal experience in the field and a high level of commitment to improving the knowledge of and response to TBE.1
Epidemiology of TBE from the public health perspective
As outlined in more detail in Chapters 3, 11, and 12 of this book, TBEV is mainly transmitted through tick bites. Food-borne infections through unpasteurized milk and milk products have no major impact in terms of epidemiology but are of increasing importance due to the growing popularity of more ‘natural’ (unprocessed and raw/ unprepared) foods. In contrast to the otherwise sporadic cases, food-borne TBE-infections occur as outbreaks with sometimes high numbers of cases (see chapter 11). Consequently, these types of TBE infection occur even in western European countries. This has become a major public health debate pitting ‘healthy food’ activists and enthusiasts against health officials with obligations to enforce food regulations. Thus, governments are challenged to find solutions.
Most natural TBE foci are well described, but new TBE affected areas have recently emerged (e.g., Japan, The Netherlands and UK in 2019, respectively; see Chapter 12b). Roughly 3,200-12,000 tick -borne encephalitis (TBE) cases are reported annually from countries where the disease is endemic,2,3 but this figure is believed to be a significant underestimation of the actual number. TBE has also become an international public health problem because of the increasing mobility of people traveling to risk areas. Today, the risk of infection is especially high for all people living in, going through (and having a stop-over) or visiting endemic areas who pursue leisure activities outdoors, and TBEV infections may even be acquired in city parks. In most regions, the main risk has shifted from an occupational to a leisure time health risk. As a result, over the last 30 years, a continuous increase in TBE morbidity has been observed in Europe4, and both the importance and awareness of TBE have increased in endemic areas and in the recent past in travelers, too.
Circulation of TBEV also depends on the population density of ticks and their hosts (see Chapter 3). Virus prevalence in the tick population within TBEV foci is determined by the duration of viremia in hosts because the virus is mostly ingested by ticks while engorging on a viremic host. Virus circulation in nature is also influenced by the percentage of immune hosts in a particular region.
Climate is another determinant of tick-borne disease dynamics. Even if major discrepancies in annual TBE incidences cannot be explained by recorded temperature increases alone, the seasonal shifts in reported cases of TBE in central and northeastern Europe suggest that TBEV transmission dynamics have changed – perhaps as a result of warmer temperatures and changes in humidity5. In addition the density of rodents (esp. those feeding on beech nuts which again is related to climate [change]) seems to be positively correlated which TBE case counts. Of note, a much higher percentage of TBE-positive individuals (whether locals or travelers) has been observed among risk groups6 such as
- individuals working in agriculture and forestry
- hikers, ramblers, joggers, and other people engaged in outdoor sports
- foragers of mushrooms and berries
- anyone who spends time outdoors (e.g. having a picnic, walking, gardening, dog-walking, or sunbathing on the grass).
Today, most people (90%) in Europe who will ultimately develop TBE visit endemic areas in pursuit of recreational activities. In central Europe and the Baltic states, recent increases in TBE may have arisen largely from changes in human behaviour that have brought more people into contact with infected ticks7 (e.g. mountain biking, playing golf or jogging instead of playing tennis). Infection with TBEV may also happen at home when infected ticks inadvertently are brought in with harvested items from the outdoors (e.g., wildflowers or Christmas trees) on clothing, or by domestic animals (e.g., dogs).8 Moreover, TBEV infections are increasingly reported to occur in gardens – even in urban areas.
TBE affects all age groups. The severity of the disease increases with age. Older generations and retired people are more active today and especially at risk of acquiring TBE. This is especially true for elderly travelers (both domestic as well as from other regions) since Europe is generally considered a safe destination requiring no specific preparation, and that can meet the needs of elderly people or those with chronic or underlying illnesses – including those that depend on a “high-standard medical infrastructure”.
In children, too, TBE can run a severe course and may lead to permanent sequelae (see chapter 6). Retrospective studies have shown TBE infection to occur in infants as young as 3 months.9 A higher incidence of TBE has been reported in boys (boy: girl ratio 7:3), who more often show signs of focal encephalitis.10
General aspects of TBE prevention
No therapy, and specifically no antiviral agent, is available against TBEV. Control of reservoir animals and of ticks is not feasible and/or has limited to no impact on TBE incidence. Prevention thus relies on 1) avoidance of exposure and 2) vaccination. Success of vaccination is based on TBE awareness among those at risk and – perhaps more importantly – those counseling them. A key challenge for public health authorities is to encourage precaution without causing alarm.11
Primary prophylaxis
Behavior
Since ticks may transmit diseases other than TBE (borreliosis being most common in TBE endemic regions), the avoidance of exposure to ticks is crucial. Not entering TBEV-endemic areas would be the safest way to avoid any risk of TBE infection. This may be an option for travelers, but it does not solve the problem for the population living in TBEV- endemic areas. For anyone entering endemic areas, the TBE risk can be reduced by personal behaviour like not running or walking through high grasses or on narrow paths that present repeated and unavoidable contact with bushes during seasons and in areas with tick activity. Persons at risk should be aware of the fact that ticks transmitting TBE often are so-called “questing ticks” (in contrast to some tropical species which are hunting ticks) and that a contact time of 0.1 second is sufficient for the attachment of ticks to the skin.
Additional recommendations (below) also may reduce the risk for TBE.
Protective clothes and repellents
- As ticks attach to any spot on the host and from there try to reach an uncovered part of the skin, adequate clothing may help to make access to the skin more difficult for ticks. Protective clothes must be completely closed to be really effective, but this may not be accepted by people spending their leisure time or holidays in endemic areas during the warm season.
- If we apply terminology strictly, then discriminating between types of repellents is important. In the narrow sense (s.s.) repellents include formulations that repel (keep off arthropods like ticks), while insecticides act as neurotoxic agents that paralyze or even kill arthropods after contact. The expression ‘repellent’ in the broad sense (s.l.) combines both means of action and will be used henceforth for simplicity.
For the impregnation of clothes, permethrin or other pyrethroids are recommended. The impregnation of clothes usually provides long-lasting protection (weeks to months), even though the solutions typically used for soaking clothes are water-based. For skin impregnation, products with proven efficacy like N-diethyl-3-methylbenzamide (formerly N, N-diethyl-m-toluamide / DEET; in higher concentrations, i.e., preferably >20%), (p)icaridin or p-menthane-3, 8-diol (PMD) are recommended. The efficacy of cutaneous repellents decreases in a comparably short time (a few hours at maximum), which in addition to chemical characteristics depends on factors such as the concentration of the chemical compound, the user’s degree of sweating, and environmental moisture. Whereas the water solubility of these products primarily might be judged as a disadvantage, this quality allows quick removal from skin or mucous membranes should they become contaminated un-intentionally.
Vector control
As with other vector-borne diseases, strategies to reduce vector density have been implemented in the past. From the beginning of the 1950s to the end of the 1970s, this was the leading strategy of TBE prevention in Russia.12 However, these large-scale control measures using tetrachlorvinphos, DDT, or Hexachlor did not produce the desired effect: no significant impact was observed on human infections. Since the virus persists not only in ticks, but also in a large number of wild animals, particularly small mammals, such measures are unlikely to eradicate or even control the disease.
Secondary prophylaxis
(Early) tick detection and removal
Ticks do not immediately penetrate the skin of the host. Some time is always required until the tick finds the most appropriate location for its bite. After the tick bite, TBEV is immediately transmitted to the host by means of the tick’s saliva. Even if the tick is already firmly attached to the skin, early removal is still advised to help to avoid other potential infections like those with Borrelia spp., where transmission of bacteria takes place between 1 and 3 days after the tick has attached itself to a human host. Thus, if a tick is detected and immediately removed after attachment, the risk of certain infections in humans is reduced substantially.13
Tick removal should follow a number of rules: screening the body after outdoor activities is always an important first measure. Adherent ticks should be removed as atraumatically as possible (https://amp.usatoday.com/amp/ 2189393002).
This can be done using a fine-tipped tweezer, long fingernails, or especially notched cards. The key is to pick the tick at the part closest to the skin and to tear it off without rotation and without squeezing the body, which could result in an increased influx of pathogens. Not recommended are any attempts to drown a tick by bathing or using ‘home remedies’ like suffocating a tick with a drop of glue, nail polish, or oil, or burning it with a match or lit cigarette. According to most authors, any advantage offered by the seemingly easier removal of the tick is by far outweighed by the disadvantage of an increased burden of infectious particles being released while the tick is struggling to death.
To overcome another misconception: if a black dot should happen to remain in the wound after tick removal, this is not the head of the tick but some part of the biting apparatus only. Taking into account the anatomy of the tick as an arachnid, the head and (in the case of TBEV), the salivary glands are sources of infection Once these are safely removed by the recommended actions and even if these resulted in incomplete removal, the window of TBEV transmission certainly would be closed.
In summary, all preventive measures described above and directed against ticks offer limited protection, only. This reinforces the need for immunological, i.e. vaccine-induced protection. A summary of prevention recommendations is provided in Table 1.
Cost-benefit analysis of TBE vaccination
Economic evaluation of TBE vaccination has become an increasingly important step in the process of including TBE vaccination in the immunization programs and/or making recommendations. However, there are only a few cost-effectiveness evaluations of the TBE vaccine.
In 1981, an overall TBE vaccination campaign was introduced in Austria14 which ultimately resulted in a substantial reduction of TBE cases.15 The economic benefit (reducing costs for inpatients care, loss of productivity and premature retirement) of that campaign was evaluated to be EUR 24 million for the years 1981 to 1990 and EUR 60 million between 1991 and 2000.16,17
A Slovenian study showed cost-effectiveness of TBE-vaccination from a healthcare payers perspective, when starting vaccination at the age of 18 years and continuing up to 80 years of age.16
In Estonia vaccination of persons ≥50 years of age is calculated to be cost-effective from the health care payer’s perspective. However, the authors stated that vaccination of the older population only has a limited impact on incidence reduction in the total population.
In 1996, a crude estimation of cost effectiveness of TBE-vaccination in the Stockholm area was done, and it was calculated that based upon the TBE-incidence at that time as well as on the costs of vaccination, mass vaccination would be an unrealistic alternative.18 However more than 20 years later much higher incidences in the unvaccinated population are reported. A health economic analysis in Sörmland County, a highly TBE-endemic area adjacent to Stockholm County, calculated that the costs per QALY (quality adjusted life year) for a fully free of charge vaccination program would come much closer to the generally acceptable cost-effectiveness threshold in Sweden. The authors therefore concluded that introducing a structured vaccination program will be cost effective at all ages, but it would be specifically more cost effective if it started in childhood.19
Such analyses are mainly based on a health care perspective, and the program would compete with other resources in the health-care sector. Therefore it is important to establish the long-term costs and health outcomes of a local TBE vaccination strategy in order to understand if funding of a TBE vaccination program yield better health outcomes at a reasonable cost.20 Differences in the underlying assumptions and disease modelling approaches as well heavily influence the outcomes of such analyses too as shown for TBE vaccination (see Fafangel 201621 – versus Smit 201522). Moreover, TBE can be associated with a high productivity loss beyond the health care sector. Increasing vaccination and age groups can be the most effective and efficient strategy to reduce the burden of TBE and protect the whole population health.10 Considering those consequences one may thus be in favour of a vaccination program or at least a vaccination recommendation. Out-of-pocket costs may have a positive impact on individual´s private consumption that is not included in the analysis from a health care perspective.
Although cost-benefit analyses are often closely linked to official recommendations for vaccination,20 this aspect is of limited value when it comes to a disease that often leads to chronic sequelae and even death but on the other hand can be easily prevented. Here, ethical considerations are the main issue. This is especially the case in affluent societies where economic resources and systems for prevention are readily available.
Table 1: General primary and secondary preventive measures
| Measure | Comment | |
|---|---|---|
| Behavior | Avoid tick-infested areas | Whenever possible |
| Avoid unpasteurized dairy products | ||
| Adhere to personal protection measures when working with viable TBEV | ||
| Clothing | Light-colored clothing that covers arm and legs (long-sleeved shirts - tight at the wrists, long pants - tight at the ankles and tucked into the socks); shoes covering the entire foot | Dark clothing is proven to be more attractive for ticks (which in addition are more difficult to identify on a dark background) |
| Use of repellents | Apply adequate repellent (with proven action against ticks) to clothing and skin | e.g. DEET in higher concentrations, (p)icaridine as well as permethrin / pyrethroids are proven to act against ticks; allow clothing to dry up before wearing |
| Early detection | Adults should be checked daily; children should be checked more frequently, i.e. after some hours of exposure (could result in 2 to 3 checks per day) | The checks should especially focus on waist bands, sock tops, under arms, other moist areas (for children: head and especially behind the ears); even adults may need the assistance of a second person to check the whole body |
| Early removal of ticks | Remove tick as soon as possible using fine-tipped tweezers or special cards (resembling carved credit cards); grasp the tick firmly as close to the skin as possible and simply tear it out without squeezing or rotating the tick | Don´t suffocate the tick (oil, cream, nail polish, water); don't burn the tick; don´t apply home remedies; don´t wait for medical services if not promptly available |
Recommendations for TBE vaccination
Recommendations for TBE vaccination vary considerably across the countries in which TBEV foci are found (see also Chapter 12a). In areas where TBEV is highly endemic and where the average pre-vaccination incidence of clinical disease is >5 cases/100,000 persons per year, the World Health Organization (WHO) and the European Centre for Disease Prevention and Control (ECDC) both recommend that vaccination be offered to all age groups, including children.24 However, this is always dependent on the evidence known so far, on the quality of the surveillance system, and does not necessarily reflect real changes in risk areas which have occurred in the past few decades. The changing epidemiology of TBE includes increasing TBE infection rates outside known endemic areas mostly north and south; case-based discoveries of new TBE foci (e.g. The Netherlands and the UK) and new areas with TBEV identification in ticks; TBEV transmissions in higher altitudes; and changed nutrition behavior resulting in an increase of risky eating habits (such as the consumption of raw milk and other raw dairy products). Furthermore, experience in several countries has shown that the recommendation to vaccinate risk groups only has no substantial impact on the annual TBE incidence. This is exemplified by the Austrian experience, where an Austria-wide vaccination campaign was started in 1981 targeting the general population in contrast to vaccinating so-called at-risk persons before, only. Subsequently the vaccination coverage of the Austrian population increased and the TBE disease numbers were drastically reduced. (see Figure 1)
The documented increase in non-vaccinated persons may be due to an increase in outdoor leisure activities as well as the fact that an increasing proportion of the population is more mobile and therefore moves from non-risky to risky areas on a frequent basis.
TBE awareness and vaccination rates
Awareness promotion is the key element in TBE control, in combination with vaccination of the general public, starting with specific risk groups, e.g., forestry workers, hunters, and military personal. The results from a cross-sectional study involving 11 European countries showed:25
- Overall awareness of TBE (83%) was lower than awareness of influenza (98%) or measles (92%). Of all respondents, 68% were aware of the TBE vaccine, and 25% had received >=1 vaccination(s) against TBE.
- Vaccination rates for TBE were lowest in Finland and Slovakia (up to10%). Much higher vaccination rates were seen in Latvia and Estonia with 53% and 31%, respectively, and highest in Austria (85%). In German endemic areas, vaccination rates varied widely (20-80%) with highest rates in a few regions like the Odenwald, where vaccine uptake even approaches 100%.
- Compliance among respondents who received >=1 TBE vaccination(s) was 61%. First and second booster injections were received by 27% and 15% of respondents, respectively.
- Strongest motivators for vaccination were fear of TBE (38%) and residence/spending time in high-risk areas (31–35%). Main reasons for not receiving vaccinations were the belief that vaccination was unnecessary (33%) and that there was no risk of contracting TBE (23%).
One of the main aspects in issuing recommendations and creating awareness is the definition of a ‘risk area.’ The Robert Koch-Institute in Germany, for example, defines and recommends vaccination for a ‘high-risk area’ as follows: wherever the TBE incidence over a floating 5-year period is significantly higher than 1/100,000 population.26 Austria, on the contrary, does not restrict vaccination recommendation to a numeric incidence. Any person living in or traveling to an endemic area is ‘at risk’ and should be vaccinated. For details on vaccination recommendations in European countries, see Chapter 12a.
The Austrian example: A success story
Austria is the only European country that implemented as early as 1981 an annual, more or less nationwide TBE awareness and vaccination campaign that targets the whole population; this has led to a substantial decline in the number of TBE cases in Austria. The Austrian example shows that containment of TBE is feasible by mass vaccination. In the pre-vaccination era, Austria had a very high recorded morbidity of TBE – probably the highest in Europe at the time, even despite some shortfalls in the notification system. In high-risk areas, the average annual incidence in the population exposed to ticks in their working environment was 0.9 per 1,000.6
The vaccination rate against TBE in the general population is 82%, which is the highest worldwide. A high awareness of TBE among the Austrian population was achieved through an annual social marketing program, and the widespread use of effective and well-tolerated vaccines has led to a successful containment of the disease. The vaccination coverage increased from 6% in 1980 to 82% in 2013 and exceeds 90% in some high-risk areas. This has led to a steady decline in the number of TBE cases from several hundred cases to roughly 50–100 cases per year (see Figure 1).24
Figure 1: TBE – annual disease numbers and vaccination rate in Austria
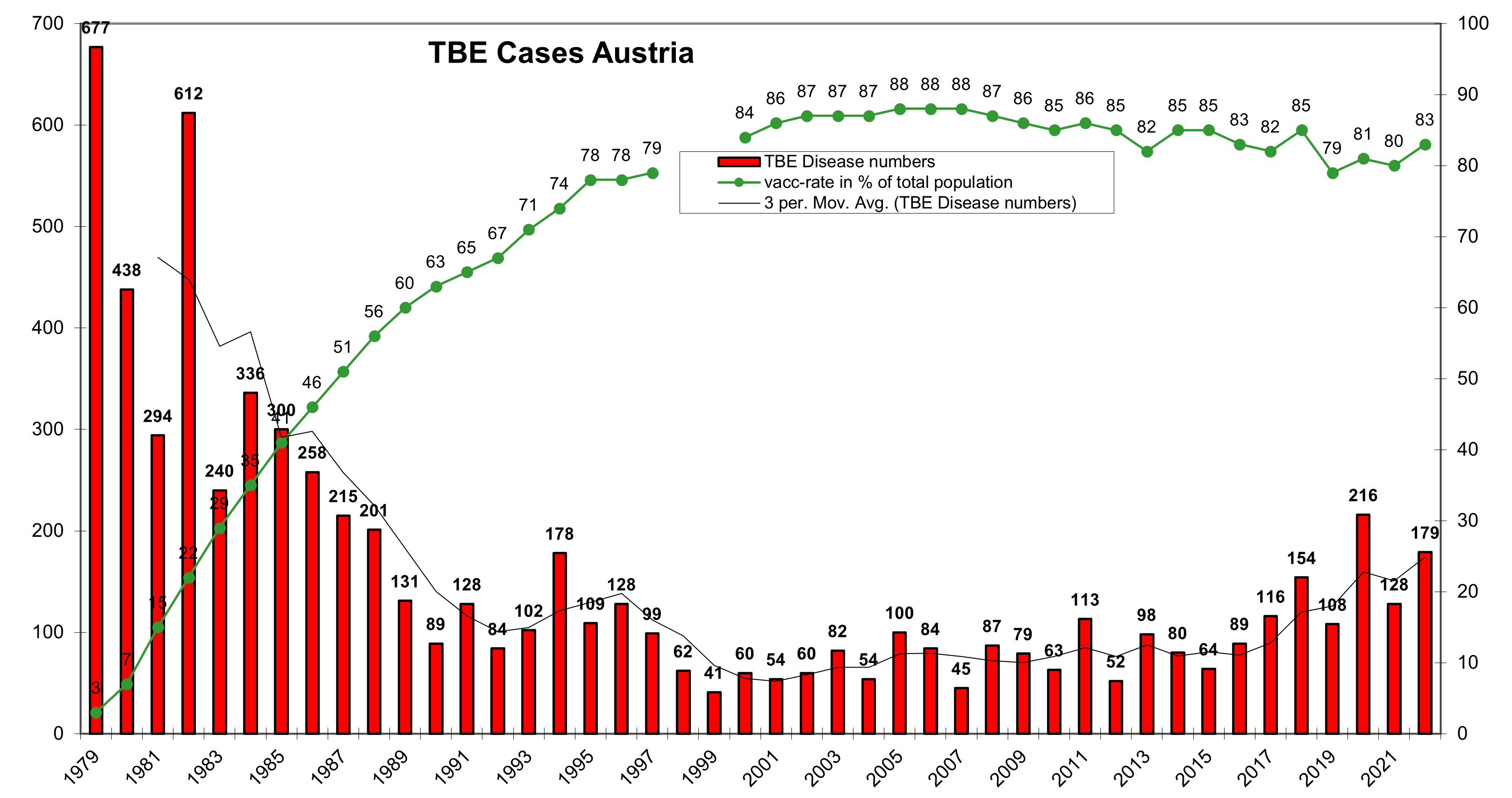
Click the image above to enlarge
The risk of acquiring TBE in an endemic area like Austria equals 1:10,000 per month of stay, and this is comparable to the risk of acquiring typhoid fever for an unvaccinated tourist in a highly endemic area like India (1:3,000 to 1:25,000).27 In fact, for an unvaccinated tourist staying in a highly endemic area in Austria for 4 weeks, the estimated risk of acquiring TBE is about 1 per 830 person-years of exposure. Based on the number of tourist overnight stays in Austria, this would equal 60 travel-associated TBE cases each summer.28
Residents of and travelers to TBE endemic areas who are at risk of tick bites are advised to receive TBE vaccination.29
The Austrian example shows that the population is willing to spend money on prevention – if aware of the problem and properly informed. This is clearly the task of local governments. In Austria, only certain highly exposed risk groups are financially supported and receive complete or partial coverage for TBE vaccination (mostly those with an occupational risk of exposure). The great majority of vaccinees in Austria invests out of its own pockets.20
Summary and recommendations for public health
In summary and to adequately address public health issues related to TBE moving forward, the authors recommend the following:
- Public health officials should make TBE a notifiable disease and establish appropriate tools for detection and reporting of human cases in their countries.
- Maps indicating TBE risks should not solely be based on incidences, since these are biased due to underdiagnosis, temporal changes, reporting structures, vaccine uptake, and other factors. If incidence maps are used, maps with known areas of TBEV presence should also be published.
- Travelers to TBE-endemic regions should be informed about TBE (even if no vaccine is available).
- Measures on how to avoid tick exposure should be publicized.
- In endemic areas, public health authorities need to effectively publish warnings that unpasteurized milk and dairy products may result in TBE infection. Laws for food safety must be implemented accordingly with respect to TBE risks.
- In endemic countries awareness campaigns on TBE, as well as vaccination campaigns should be established.
- ID specialists in non-endemic areas dealing with international travellers should update their knowledge (e.g. by reading the comprehensive chapter on TBE in Netter’s Infectious Diseases) and include TBE as a differential diagnosis whenever necessary.
Contact
michael.kunze@meduniwien.ac.at
Citation
Kunze M, Erber W, Haditsch M. TBE as a matter of public health. Chapter 13. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi: 10.33442/26613980_13-6
References
- Kunze U. The International Scientific Working Group on Tick-Borne Encephalitis (ISW TBE): Review of 17 years of activity and commitment. Ticks Tick Borne Dis. 2016;7(3):399-404.
- ECDC. Tick-borne encephalitis – Annual Epidemiological Report for 2018. 2019.
- Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86(24):241-56.
- Süss J, Klaus C, Diller R, Schrader C, Wohanka N, Abel U. TBE incidence versus virus prevalence and increased prevalence of the TBE virus in Ixodes ricinus removed from humans. Int J Med Microbiol. 2006;296 Suppl 40:63-68.
- Randolph SE. Evidence that climate change has caused ’emergence’ of tick-borne diseases in Europe? Int J Med Microbiol. 2004;293 Suppl 37:5-15.
- Kunz C. Vaccination against TBE in Austria: the success story continues. Int J Med Microbiol. 2002;291 Suppl 33:56-57.
- Randolph SE. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe.. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):1045-1056.
- HC. H. Neurologische Komplikationen nach Zeckenkontakt. Deut Aerztebl. 1980;77:2063-6.
- Grubbauer HM DH, Spork D, Zobel G, Trop M, Zenz D. . Tick-borne encephalitis in a 3-month-old child. Eur J Pediatr 1992;151:743-4.
- Cizman M RR, Zakotnik B, Pokorn M, Arnez M. Severe forms of tick-borne encephalitis in children. Wiener Klin Wochenschr. 1999;;111:484-7.
- Slunge D, Jore S, Krogfelt KA, Jepsen MT, Boman A. Who is afraid of ticks and tick-borne diseases? Results from a cross-sectional survey in Scandinavia. BMC public health. 2019;19(1):1666.
- Uspensky I. Ticks as the main target of human tick-borne disease control: Russian practical experience and its lessons. J Vector Ecol. 1999;24(1):40-53.
- Kahl O, Janetzki-Mittmann C, Gray JS, Jonas R, Stein J, de Boer R. Risk of infection with Borrelia burgdorferi sensu lato for a host in relation to the duration of nymphal Ixodes ricinus feeding and the method of tick removal.Zentralbl Bakteriol. 1998;287(1-2):41-52.
- Kunz C. TBE vaccination and the Austrian experience. Vaccine. 2003;21 Suppl 1:S50-55.
- Heinz FX, Stiasny K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis.Microbiol Mol Biol Rev. 2017;81(1).
- Smit R. Cost-effectiveness of tick-borne encephalitis vaccination in Slovenian adults. Vaccine. 2012;30(44):6301-6306.
- Schwarz B. Health economics of early summer meningoencephalitis in Austria. Effects of a vaccination campaign 1981 to 1990. Wien Med Wochenschr (1946). 1993;143(21):551-555.
- Haglund M, Forsgren M, Lindh G, Lindquist L. A 10-year follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy.Scand J Infect Dis. 1996;28(3):217-224.
- Askling HH, Insulander M, Hergens MP, Leval A. Tick borne encephalitis (TBE)-vaccination coverage and analysis of variables associated with vaccination, Sweden. Vaccine. 2015.
- Shedrawy J, Henriksson M, Hergens M-P, Askling HH. Estimating costs and health outcomes of publicly funded tick-borne encephalitis vaccination: A cost-effectiveness analysis. Vaccine. 2018;36(50):7659-7665.
- Fafangel M, Cassini A, Colzani E, et al. Estimating the annual burden of tick-borne encephalitis to inform vaccination policy, Slovenia, 2009 to 2013.Euro Surveill. 2017;22(16):30509.
- Smit R, Postma MJ. Review of tick-borne encephalitis and vaccines: clinical and economical aspects. Expert Rev Vaccines. 2015;14(5):737-747.
- Smit R. Reviewing estimates of the burden in disability-adjusted life years (DALYs) of tick-borne encephalitis in Slovenia. Expert review of pharmacoeconomics & outcomes research. 2019:1-5.
- Kunze U, Kunze M.The Austrian Vaccination Paradox: Tick-borne Encephalitis Vaccination Versus Influenza Vaccination. Cent Eur J Public Health. 2015;23(3):223-226.
- Erber W, Schmitt HJ. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick Borne Dis. 2018;9(4):768-777.
- Koch-Institut R. Epidemiologisches Bulletin. Vol No. 17, 27. 2017; available at: http://www.rki.de/DE/Content/Infekt/ EpidBull/Archiv/2017/Ausgaben/17_17.pdf?blob=publicationFile.2017.
- Rendi-Wagner P. Risk and prevention of tick-borne encephalitis in travelers. .J Travel Med. 2004;11(5):307-312.
- Kunze U. Is there a need for a travel vaccination against tick-borne encephalitis? Travel Med Infect Dis. 2008;6(6):380-383.
- ECDC. Key messages about tick-borne encephalitis and tick-borne diseases. https://wwwecdceuropaeu/en/tick-borne-encephalitis/facts/key-messages. 2020.