Key Points
- Worldwide there are 6 different TBE vaccines – two from Western Europe, three from Russia and one from China. The two western European vaccines and one of the Russian vaccines have an adult and a pediatric formulation.
- The products names are FSME IMMUN and FSME-IMMUN Junior; Encepur adults and Encepur children, Klesch-E-Vac, EnceVir and EnceVir Neo, Dry lyophilized TBE Moscow and Sen Tai Bao
- All TBE vaccines except the one from China have similar but not identical immunization schedules with primary immunization (>3 doses) and regular booster vaccinations. For FSME-IMMUN, Encepur and EnceVir rapid immunization schedules are also licensed. The Chinese vaccine is given with 2 primary doses 2 weeks apart followed by annual boosters.
- All vaccines induce significant immune responses. In the absence of a formal correlate of protection, the presence of neutralizing antibodies is used as a surrogate marker for protection.
- Recent clinical studies show long-term seropersistence of TBE antibodies after the first booster vaccination (dose 4) with the two European vaccines.
- An effectiveness of approximately 99% (years 2000–2006) and 98.7% (years 2000-2011) was calculated for regularly vaccinated persons in Austria, a country with established high vaccination uptake.
- Whereas in Western Europe post-exposure prophylaxis with immunoglobulins was discontinued in the late 1990s, in the highly endemic regions of Russia it continues to be common practice.
- Both – FSME-IMMUN and Encepur are well tolerated with a well-established safety profile. TBE-Moscow and EnceVir appear to be somewhat more reactogenic.
Active immunization
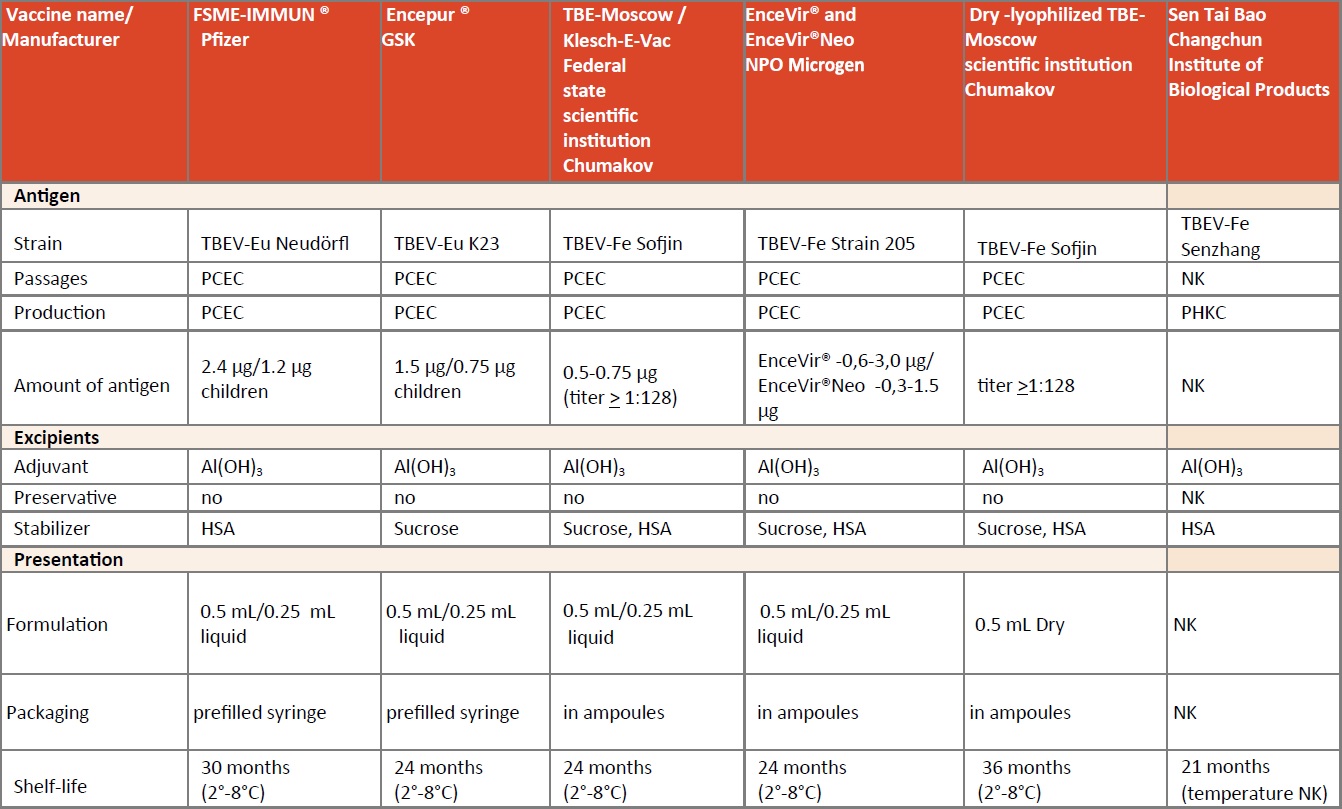
The first generation of TBE vaccines was produced in Russia. These vaccines were based on the TBEV-FE strain Sofjin, and were mouse-brain propagated. Over several decades, formulations and growth media were adapted step-by-step to result in the currently used TBE vaccines, details of which are summarized in Table 1. The two so-called ‘Western vaccines’ are FSME-IMMUN, which is licensed through the mutual recognition procedure (MRP) of the European Medicines Agency (EMA), and Encepur, which has several national licenses. These two vaccines are distributed mainly in Europe and Israel, while the other TBE vaccines are predominantly produced for local markets.
Manufacturer and products
TBE vaccines are produced commercially by five manufacturers. Two are produced in Europe, one by Pfizer (Vienna, Austria), one by GSK Vaccines (Marburg, Germany; bought by Bavarian Nordic, Kvistgaard, Denmark end 2019); 2 in Russia: IPVE (Moscow, Russia) and Microgen (Tomsk, Russia); and one in China: Sen Tai Bao (Changchun Institute of Biological Products Co., Ltd.; CIBP). The two manufacturers in Europe use very similar manufacturing processes but different virus strains and stabilizers. Both of them have licensed formulations for adults (Pfizer: FSME-IMMUN; Bavarian Nordic: Encepur) and for children older than one year (Pfizer: FSME- IMMUN Junior; Bavarian Nordic: Encepur-Children). FSME-IMMUN Junior is licensed for children up to and including 15 years of age, whereas Encepur-Children is licensed up to and including twelve years of age. In some countries, FSME-IMMUN is marketed as TicoVac. FSME-IMMUN, Enceput as well as EnceVir have (half-dose) formulations for children and the TBE-Moscow vaccine is approved for use in children age 3 years or older. Human serum albumin (HSA) is used as a stabilizer by Pfizer, IPVE, CIBP, and Microgen, whereas Bavarian Nordic uses an increased amount of sucrose for this purpose. An overview of the excipients of the European and Russian vaccines is shown in Table 1.
FSME-IMMUN
This vaccine is based on the Austrian TBE strain Neudörfl (TBEV-EU) and was licensed first in 1976. The virus was primarily passaged in the brains of specific pathogen-free (SPF) baby mice and then propagated in primary SPF chicken embryo cells. The vaccine formulation underwent several changes over subsequent decades until 2000. The actual licensed vaccine is a formaldehyde-inactivated, whole-virus vaccine (2.4 mcg antigen per dose), adjuvanted with aluminium hydroxide and containing HSA as an essential stabilizer. Details of the actual formulation are described in Table 1. A pediatric formulation containing half of the adult dose (FSME-IMMUN Junior) was licensed in 2002. The current manufacturer of FSME-IMMUN is Pfizer.
Encepur
This vaccine is based on the European subtype virus strain K23, isolated in Karlsruhe in southern Germany and originally licensed first in Germany in 1991 as Encepur by Chiron Behring, Marburg, Germany.1 Similar to FSME-IMMUN, the seed virus for this vaccine is grown on primary chick embryo cells. The virus is inactivated by formaldehyde, adsorbed to aluminium hydroxide, and contains 1.5 mcg of antigen. A pediatric formulation containing half the adult dose (Table 1) has been available since 1994.2 The genomic sequence of the K23 vaccine virus in the Encepur formulation has mutations compared to the originally published sequence.90 However, the clinical impact of the modified primary amino acid sequence is unknown. At the end of 2019 Bavarian Nordic acquired Encepur from GSK. According to communications by GSK and Bavarian Nordic, vaccine manufacturing will be transferred over the next 5 years, sales and marketing responsibility will be assumed immediately from 2020.
Russian vaccines
Three TBE vaccines have been developed and are marketed in Russia (see Chapter 12b: Russia). All of them are cultured on chick embryo cells and are formalin- inactivated. EnceVir, manufactured by Microgen, Tomsk, is based on the TBEV-FE subtype strain 205.4
There is a vaccine for adults (EnceVir (0.5) and as of 2014 also a pediatric formulation (EnceVir Neo (0.25) for children 3-17 years). Klesch-E-Vac is based on the TBEV-Fe prototype strain Sofjin, and manufactured by the Federal State Enterprise of Chumakov Institute of Poliomyelitis and Viral Encephalitides (IPVE). It is provided as a suspension for injection.3 Klesch-E-Vac has an adult (0.5mL) and also a pediatric formulation licensed for use as of 12 months to 16 years of age (half of the adult dose, i.e. 0.25 mL).
In addition, there is a dry-lyophilized TBE-Moscow vaccine (no specific trade name), based on the Sofjin strain.3 The producer is also the Federal State Enterprise of Chumakov Institute of Poliomyelitis and Viral Encephalitides (IPVE). The product is approved for use in patients from 3 years of age as a unified formulation.
Sen Tai Bao
The Sen Tai Bao (Changchun Institute of Biological Products Co. Ltd: CIBP; in Changchun, Jilin Province, China) TBE vaccine is manufactured by the Changchun Institute of Biological Products (CIBP) and marketed in China only.5 There a first vaccine against TBE was developed in 1953, by propagating the TBEV on mouse brain tissue followed by inactivation. It was an inactivated TBEV grown on infected mouse brain tissues. Between 1953 and now several vaccine formulations have been developed and used. Some of the earlier vaccines were grown on chicken embryo cells.91 The current formalin-inactivated vaccine formulation is based on the TBEV-FE strain Senzhang. The vaccine is grown on primary hamster kidney cells, uses HSA as the stabilizer and aluminum hydroxide as adjuvant. This vaccine has been approved for use in adults and children 8 years of age or older since 2004.6
Vaccination schedules
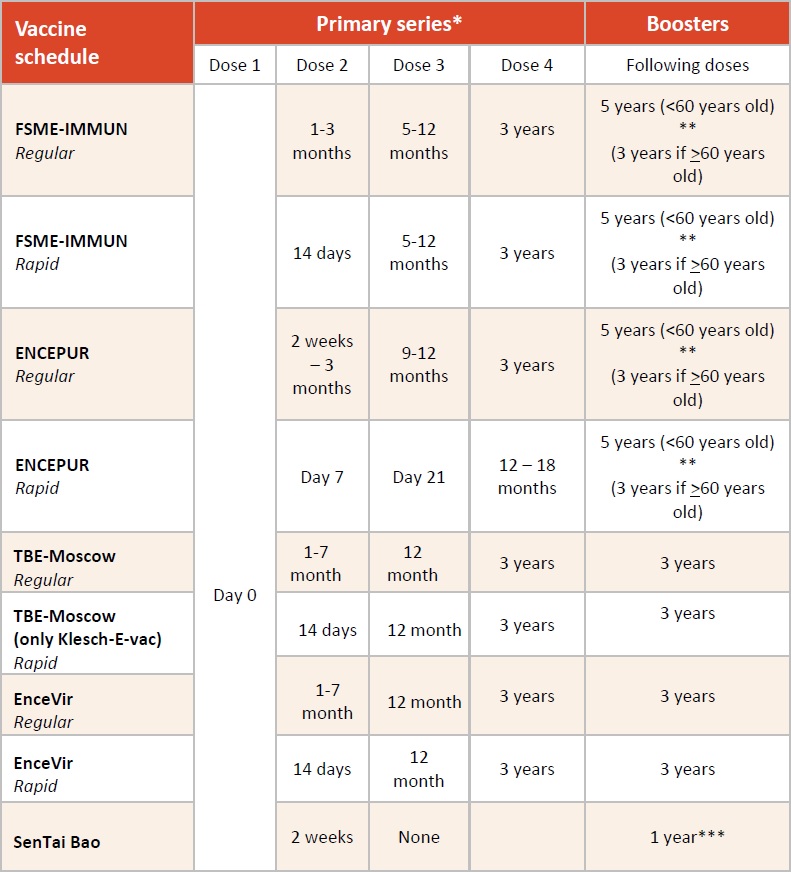
Details on the schedules for the different licensed vaccines are summarized in Table 2. In brief, the basic immunization protocol for all vaccines consists of 3 doses (except the Sen Tai Bao, which has only 2 doses), similar to conventional immunization schedules with other aluminum-adjuvanted, inactivated vaccines: the first vaccination is followed by a second shot 4-12 weeks later, and a third shot is administered 5-12 months later. However, considerable differences still exist between vaccine brands. For Encepur and FSME-IMMUN, a rapid or accelerated immunization schedule is licensed for children and adults (Table 2). In the context of the conventional immunization schedule for any of the 4 non-Chinese vaccine brands, the first TBE booster immunization is recommended 3 years following the third vaccination of the primary series. Subsequent boosters for the European vaccines are recommended at intervals of 5 years in persons below 50 and 60 years of age for Encepur and FSME-IMMUN, respectively, and every 3 years for persons older than 50 or 60 years of age, respectively. Booster doses for the Russian vaccines are recommended every 3 years for all age groups.
Contraindications and precautions
In general, for all TBE vaccines, hypersensitivity to the active substances, any of the excipients, or production residues constitutes a contraindication to immunization (Table 1). For the four non-Chinese TBE vaccines, severe hypersensitivity to egg, chicken proteins, or latex may cause severe allergic reactions in sensitized individuals. A moderate allergy to egg proteins (defined as hives after consumption/injection) does not constitute a contraindication for TBE vaccination with either vaccine. However, patients with moderate egg allergy should be monitored for one hour after application. Therefore, persons with proven “non-severe egg allergy” can receive a TBE vaccination. In case of a moderate or severe acute illness with or without fever, TBE vaccination should be postponed.
Previous exposure to other flaviviruses or flavivirus vaccines (for example, against Yellow fever [YF], Japanese encephalitis virus [JEV], or dengue virus) has been suggested to affect the immune response to TBE vaccination. While for a long time this was not adequately studied in humans, a new study became available in 2019101, which investigated the influence of pre-existing YF vaccine-derived immunity on the antibody response to TBE vaccination. By comparing samples from YF pre-vaccinated and flavivirus-naive individuals, it could be shown that YF immunity not only caused a significant impairment of the neutralizing antibody response to TBE vaccination but also a reduction of the specific TBE virus neutralizing activities (NT and ELISA-titer ratios). Although the clinical relevance of these findings remains unclear, in practice, an increased awareness of the possible impact of pre-existing flavivirus immunity in the assessment of flavivirus vaccines appears to be warranted.In contrast, TBE vaccination has been shown to enhance the immune response to an inactivated JEV vaccine,7 but even though cross-reactive antibodies have been described, there is no evidence of actual cross-protection between JEV and TBE vaccines.
For both European TBE vaccines, there is no data on their use during pregnancy and lactation. As with all other inactivated vaccines, vaccine administration during pregnancy may be considered after carefully weighing risk and benefit.
Vaccine stability and storage
FSME-IMMUN is available as a pre-filled syringe without needle. The vaccine must be refrigerated at 2°C to 8°C. The shelf life is 30 months. Encepur is available as a pre-filled syringe with and without needle and must be stored at the same temperature (between 2°C and 8°C). The shelf life is 24 months. TBE-Moscow vaccine has a shelf life of 24 months and EnceVir of 36 months, both with the same temperature requirements as the European vaccines. The currently licensed Chinese vaccine has a shelf life of 21 months.
Vaccine immunogenicity
No clinical studies with efficacy endpoints have been conducted on any of the licensed TBE vaccines. These vaccines have been registered on the basis of immunogenicity and safety studies, which consistently show strong immune responses after primary vaccination with the vaccine. A Cochrane Collaboration review published in 2009 summarized 11 randomized clinical trials (10 publications), conducted with 3 different TBE vaccines (IPVE, FSME-IMMUN, and Encepur) and involving 8,184 subjects (6,586 adults and 1,598 children).8 Overall seroconversion rates exceeding 87% were observed. Studies conducted by the respective manufacturers report seroconversion rates in the range of 92%–100% for Encepur and FSME-IMMUN, as measured by a commercialenzyme-linked immunosorbent assay (ELISA) or neutralization test (NT), with seroconversion being defined as NT ≥ 1:10, or according to the recommendations of the ELISA manufacturer.9-12
FSME-IMMUN
The clinical development program for FSME-IMMUN included 13 studies that investigated the immunogenicity and safety of the vaccine in approximately 5,180 adults and 6,430 children. An additional 4 studies on FSME-IMMUN were identified after review and analysis of published literature.9 The seroconversion rate in adults 16 to 65 years of age, vaccinated according to the conventional schedule, was 97% after the second dose and ranged between 99.5% and 100% after the third dose, as measured by ELISA and/or NT.9 When the rapid immunization schedule (Table 2) was used, seroconversion rates in NT after the second vaccination were 98.0% and 89.9% in adults younger or older than age 50, respectively, and 100% and 99.3% in those 2 age groups after the third vaccination, respectively. Two pediatric studies (a dose-finding study with more than 400 children who received the later licensed pediatric dose and a large safety study with an immunogenicity subset that included approximately 370 children, all between the ages of 1 and 15 years) found seroconversion rates (ELISA) of 96% to 100% (depending on the age sub-group) after the second vaccination and almost 100% in all age subgroups after the third vaccination.13
Table 2: Immunization schedules for TBE vaccines according to WHO recommendations
Dose 1 considered to be given on day „0“, intervals in table below given in months unless stated otherwise. Please note that „rapid schedules“ are not licensed for children.
Click the image above to enlarge
Another pediatric study investigated immune response in 149 and 152 children 1–11 years of age, who were vaccinated with FSME-IMMUN Junior and Encepur Children, respectively, in the context of a primary immunization schedule. According to the NT based on the Neudörfl strain, seropositivity rates after the second vaccination in the combined age groups was 100% in children who received FSME-IMMUN Junior and 97.8% in those who received 2 vaccinations with Encepur Children.14 A third vaccination with FSME-IMMUN Junior induced 100% seropositivity in both study groups.15
An earlier pediatric study, which investigated the immune response in 334 children to both FSME-IMMUN Junior and Encepur Children for the first two vaccinations, using the conventional as well as the rapid immunization schedule, found higher seropositivity rates (NT ≥ 10) in the Encepur-immunized group versus the group that received FSME-IMMUN Junior, using either vaccination schedule. Upon completion of the primary vaccination course, and after the third dose (given with Encepur Children), >95% of all children achieved an NT ≥ 10.16 Both studies confirmed the interchangeability of the two TBE vaccines when given as a third dose in the context of a conventional or rapid primary immunization schedule.
Encepur
Data on the immunogenicity of Encepur from eight clinical and post-marketing studies, which included 7,500 subjects, showed 100% seroconversion or a 4-fold rise in anti-TBEV antibodies after primary immunization.17 Similar immunogenicity was achieved with either conventional or rapid immunization schedules (see Table 2).12
In 3 studies, comprising a total of 3,118 subjects between ages 12 and 76 years, the non-inferiority of the new polygeline-free formulation to the former vaccine containing polygeline was demonstrated.18 In addition, the rapid immunization schedule using the new formulation was investigated.17,19,20 The new formulation was also shown to be safe and immunogenic in a review of data from clinical trials and post-marketing experience in approximately 7,500 subjects aged 1 to 77 years.20 The immunogenicity of the vaccine and the advantages of the rapid immunization schedule were further confirmed in a number of pediatric trials that enrolled more than 3,500 children 1–11 years of age.21,22 The immunogenicity of the rapid schedule in children, as well as the interchangeability with FSME-IMMUN when given as a third dose, was shown by Wittermann et al.23 Seropositivity rates of 99% and 100% were determined at 3 and 5 years, respectively, after booster doses in children 1–11 years of age.16
Russian vaccines
The Russian vaccines, TBE-Moscow (Klesch-E-Vac) and EnceVir, have been evaluated in 2 clinical studies, each involving 200 adults. Antibody titers ≥1:80 (hemagglutination inhibition [HI] test) were detected following 2 doses, 2 or 5 months apart, in 84% and 93% of subjects receiving TBE-Moscow vaccine and in 82% and 89% of the vaccinees who received EnceVir, respectively.24,25
Another study with an age-stratified analysis of 325 subjects found at least a 4-fold increase of HI-antibody titers in 96%, 93%, and 89%, respectively, for each of 3 age groups: 3–6 years, 7–14 years, and 15–18 years, after vaccination with TBE-Moscow vaccine, versus 84%, 97%, and 92%, respectively, for the same age groups after receiving the EnceVir vaccine.23
No significant differences regarding immunogenicity against different TBEV strains could be found between TBE-Moscow vaccine and FSME Immun Inject (FSMEV propagated in mouse brain cells).4 After 2 doses of the TBE-Moscow vaccine given 4 months apart, 92% of children and adolescents ages 7–17 years achieved a 4-fold rise in antibody levels compared with baseline.4 Based on these results, the vaccine was recommended first for use in children and later for use in adults.4
A study comparing EnceVir and TBE-Moscow vaccine (N=400) found seropositivity (HI test) in 82% and 89% of patients, respectively, after 2 doses of EnceVir given 2 or 5 months apart, whereas the seropositivity rates with the TBE-Moscow vaccine were 84% and 93%, respectively.26-28 Furthermore, the 2 vaccines were also compared in 325 children who received 2 doses of either vaccine. A 4-fold rise in HI titer was achieved in 84% to 97% of the children with EnceVir and in 96% to 98% with TBE-Moscow vaccine, respectively.29 Twelve months after the last dose of EnceVir or TBE-Moscow vaccine, 72% and 87%, respectively, of the vaccinated individuals were still seropositive. A booster response was efficacious in all of the 131 children who received a third dose 1 year after the first 2 vaccinations.30
In studies comparing the available Russian TBE vaccines, seroconversion rates of 59% and 83%, after 1 and 2 doses, respectively, were achieved with TBE-Moscow vaccine, versus 75% and 85%, respectively, with EnceVir.31 Even without randomized controlled efficacy trials, the field effectiveness of the 2 Russian vaccines has been proven in highly endemic regions, e.g., in Krasnoyarsk and Sverdlovsk.31-33
Sen Tai Bao
According to an English-language article summarizing five clinical studies investigating the current Chinese TBE vaccine in children 8–17 years of age (N=616), in adults <60 years of age (N≈5600), and in elderly individuals ≥60 years of age (N=166), seropositivity rates (as measured by plaque reduction neutralization test and/or ELISA) ranged between 86.4% and 98.8% after 2 doses.6 In the group of subjects ≥60 years old, the seropositivity rate 28 days after the second vaccination was 97.3%. In one of the studies, seropersistence rates of 86.5% and 76.9% were observed six and twelve months after the second vaccination, respectively.
Comparative studies
A recent randomized study compared the immunogenicity of TBE-Moscow, EnceVir, FSME-IMMUN, and Encepur Adults by using the Far-Eastern virus strain P-73 in adults.34 All vaccines induced neutralizing antibodies against the tested strain with TBE-Moscow; neutralizing antibodies were detected in 100% and 94% of the vaccinees after 2–5 months and 2 years, respectively. With EnceVir, neutralizing antibody detection rates were 88% and 84%; with FSME-IMMUN, 88.2% and 78.1%; and with Encepur, 100% and 100%, respectively.
Irregular vaccination
The question of how to address prolonged intervals between the vaccinations of the primary series or between the boosters has long been debated. An investigation of the field effectiveness of TBE vaccination in Austria – a country in which 88% of the total population is vaccinated against TBE at least once and 58% is regularly vaccinated according to the recommended schedule – found an overall effectiveness in regularly vaccinated persons of about 99%, and 95% in subjects with a record of irregular vaccination.35,36 Furthermore, in a cohort study of more than 1,100 persons whose vaccination deviated from the recommended schedule, a single booster immunization with FSME-IMMUN was administered up to 20 years after 1, 2, or 3 primary vaccinations.37 The results of this study demonstrated that, independent of the interval since last vaccination and the age of the vaccinee, a sufficient booster response was induced if at least 2 or 3 primary vaccinations were previously administered.37,38 In addition, similar results have been seen with Encepur, given as a catch-up vaccination after primary or primary + booster vaccination.51
Vaccine interchangeability
In general, it is preferred that the same vaccine brand is used for the complete primary immunization series. However, in order to not interrupt a vaccination series in case of unavailability of a certain vaccine, the immunization series can be completed with a different brand of TBE vaccine. Several studies confirmed that FSME-IMMUN and Encepur can be safely interchanged for the third vaccination in the context of the conventional primary immunization of adults and children, as well as for subsequent booster vaccina-tions.11,15,23 In two studies – one in adults and one in children aged 12 years and younger – FSME-IMMUN was administered as the 3rd dose of the primary schedule after two doses of Encepur;11,15 in a third pediatric study Encepur was given for the 3rd dose after two doses of FSME-IMMUN.23
A review describing three studies in which Encepur was given as a booster after a complete primary immunization with FSME-IMMUN (with or without booster) and further three studies in which Encepur or FSME-IMMUN was given for the third vaccination after two doses of the respective other brand in the context of the conventional schedule come to the same conclusion, irrespective of the somewhat differing immunogenicity results.92 These differences, as mentioned several times throughout this chapter, are primarily due to the different test systems used – utilizing a homologous or heterologous TBE virus strain.
A switch from Encepur to FSME-IMMUN for the 3rd vaccination of the rapid immunization schedule (1-7-21), as well as a switch between first and second vaccination in the conventional schedule for FSME- IMMUN as well as for Encepur should be considered only under exceptional circumstances, as these schedules are not licensed.
Correlates of protection
Neutralizing antibodies directed against the protein E represent the most important mechanism of protection against TBEV, not only after natural infection but also after vaccination, even if antibody responses in both cases differ.39 According to the World Health Organization (WHO), in the absence of a formal correlate of protection for TBE vaccines, these neutralizing antibodies can be used as a surrogate marker for immunity.33 Unfortunately, there is no generally accepted, standardized neutralization test nor any international reference reagents. In general, a titer =1:10 is considered seroprotective;40 however, in the context of some vaccine licensure studies, titers of =1:2 were accepted as a correlate for a significant immune response.41 Neutralization assays as used in various studies to determine seroprotection after vaccination differed to a large extent: their sensitivity differed as well as different test protocols were used, which makes a comparison of results difficult. There is only one occasion of directly comparable TBE antibody test results with standardized serum samples available and even in this study different NT test results were shown. Moreover, detection of virus-neutralizing antibodies in vitro was never correlated with serum antibody concentration in vivo necessary to achieve solid protection in a subject.
ELISA results are not suitable as reliable surrogate markers for neutralizing antibodies due to cross-reactivity with other flaviviruses (specifically antibodies resulting from infection or vaccination). Moreover, the ELISA assay does not distinguish between antibodies with low and high avidity, hence determining also antibodies without neutralizing capacity. Therefore, ELISA measurements are primarily useful for screening purposes. The HI test, which has been broadly used in the past, is no longer considered state of the art.
Cross-protection
Evidence exists that TBE vaccines protect not only against the homologous subtype, but also against heterologous subtypes (European, Siberian, and Far-Eastern TBEV subtypes). In vitro and in vivo studies have shown broad cross-neutralizing capacity of vaccine-induced antibodies by either vaccine.24,25,34,42,43
Moreover, a recently published systematic review44 supports robust cross-neutralization with the exception of 1 strain (TBEV-Fe P-69), for which a significantly lower level of neutralization was determined. In contrast, there is no evidence from human studies (except against Omsk HF)43 that vaccine-induced TBEV antibodies provide cross-protection against other flaviviruses.
To overcome the problem of missing comparability data between immune responses to different TBEV strains, due to a poorly standardized methodology, a novel test system that uses hybrid viruses was developed; this system allows an unbiased head-to-head comparison of the humoral responses against different TBEVs from all 3 subtypes. Studies using this new technique have found comparable vaccine-induced neutralizing titers against TBEVs of all subtypes, in sera of subjects who received 2 doses of FSME IMMUN Junior, and somewhat reduced, but still protective, neutralization capacity against Omsk hemorrhagic fever virus (OHFV).43 Another study found differences in the ability of 2 European pediatric TBE vaccines to induce antibodies capable of neutralizing heterologous TBEV strains.45
While it has been shown that an immunization with Encepur in subjects leaving in regions with Far Eastern TBEV circulation induced higher immune responses in originally seropositive as compared to seronegative individuals, similar data with vaccines based on the Far Eastern TBEV strains are limited.94
A recently published study found statistical significant differences in the immune response in subjects with pre-existing immunity to the TBEV FE strain Sofjin or Siberian strain Ekaterinburg-27-11-06 as compared to seronegative individuals, only after the first vaccination with one of the two Russian TBE vaccines (Tick-E-Vac based on FE strain Sofjin and EnceVir based on FE strain 205). After the second dose, the difference was insignificant95
Antibody persistence, age, and duration of immunity
Up to the year 2004, 3-year booster intervals were recommended for the two European TBE vaccines. However, in 2004 and 2006 data suggesting a longer seropersistence became available.38,46 Since then, studies investigating the seropersistence after primary and booster vaccinations with both European vaccines have been conducted.16,19,47-49
The seropersistence of TBEV antibodies in 347 adults between the ages of 18 and 67 years was evaluated two and three years after completion of the primary vaccination, with the first two doses being either FSME-IMMUN or Encepur. The third dose consisted of FSME-IMMUN for all study subjects.50 Seropositivity rates of 96.8% and 95.4% were determined using NT two and three years after the third dose of the primary series, respectively. All subjects (100%) achieved seropositivity after the subsequently administered first booster vaccination.
A subsequent long-term investigation of seropersistence after an Encepur booster vaccine was initiated,47,48,52 and seropositivity rates (SPR) were evaluated from 2 to 10 years after the booster was given. After 2, 3, and 4 years, SPR of 95.9%, 96.7%, and 93.8% were found. In subjects 50–60 and >60 years of age, SPR dropped after 4 years to 93.0% and 91.7% for the 2 age groups, respectively. After five and six years, SPR in subjects below age 60 dropped to 96% and 94%, while for subjects age 60 years and older, rates of 89% and 86% were detected, respectively. Geometric mean titers (GMTs) were also lower not only in subjects age 60 years and older, but also in subjects older than 50 years. At the end of the study, 8 and 10 years after the booster, SPR were 86.8% and 77.3%, with a pronounced age correlation, while in subjects younger than 50 years of age, seropositivity rates of 83.9% could be detected after ten years. In the age group older than 50 years, only 66% of these subjects remained seropositive.47 Similar to observations in young adults, seropersistence over a 5-year period was shown for adolescents who received their primary immunization according to different immunization schedules.16,53
A prospective investigation of seropersistence of TBE antibodies was recently published by Konior et al.88. The study – a follow-up study of the one in 347 adults described above, investigated the seropersistence of TBE antibodies up to 10 years after a primary immunization and first booster with FSME-IMMUN. The necessity for a booster vaccination was evaluated on the basis of annual NT determinations. As expected, the decrease in seropositivity was more pronounced in the elderly as compared to younger individuals – the proportion of subjects left potentially unprotected by prolonging the booster interval beyond 5 years was 7% in the 18–49 years age group and 18% in the 50–60 years age group. By 10 years, these proportions increased to 11% and 26% in the 18–49 years and 50–60 years age groups, respectively. Nevertheless, overall, a total of only 47 subjects (14.9%) received the second booster dose over the follow-up period, and 84.9% of the study subjects were still seropositive after 10 years. Seropositivity rates were even higher (88.6%) in subjects below 50 years of age.
In a phase IV follow-up study published by Beran et al.89 adults and adolescents who had received 3 different primary vaccination schedules (rapid, conventional and accelerated conventional) in a predecessor study and a booster dose 12-18 months or three years after the primary series were followed for the persistence of their TBE antibodies by annual NT determinations. Overall, ≥97% of the study subjects in the per protocol set were seropositive (NT titers =10) across all timepoints, regardless of the primary vaccination schedule, however, older age groups showed overall lower GMTs.
Long-lasting seropersistence of TBEV antibodies after the first booster was confirmed by a newly published study98 investigating the antibody persistence in children, adolescents and young adults who received their primary immunization with FSME-IMMUN Junior when they were aged 1-15 years and an age appropriate booster with either FSME-IMMUN or FSME-IMMUN Junior 4-5 years after the primary schedule. Seropositivity rates as determined by NT were 99.4% after 5 years and 90.3% after 10 years.
The seropersistence studies with both European vaccines show long-term anti-TBEV antibody persistence after the first booster vaccination, especially in the population below 50-60 years of age, as well as excellent boostability in all age groups, indicating the establishment of a strong immune memory. Whereby while the question if the permanent presence of protective levels of TBEV antibodies alone is responsible for the overall good effectiveness of TBE vaccines remains open, the rapid immunological memory response definitely contributes in this regard. In terms of comparability of study results, it should be mentioned that due to the different test systems used (different NT assays) the studies are not directly comparable. Before results on long term seropersistence became available a recommendation for a 10-year booster interval starting directly after the 3rd vaccination of the primary series was introduced in 2006 in Switzerland. The primary goal of this change was to increase the vaccine coverage, which was achieved only to a moderate extent in some Swiss cantons in the years thereafter.89 Nevertheless, the increased vaccine coverage did not cause a reduction in the incidence of TBE in the country so far. Therefore, very recently, the whole territory of Switzerland except cantons of Geneva and Ticino – is now defined as a TBE risk area, which is hoped to further increase the vaccine coverage in the country.97
Based on the meanwhile available long-term seropersistence data after the first booster a prolongation of the booster intervals appears feasible, especially for the younger population. Primarily in countries with very low vaccination coverage this could have a positive effect. However, all data generated with respect to this issue have limitations, since the study participants were fully immunocompetent, and therefore do not entirely represent an unselected population. Moreover, it is questionable if countries with very well-established vaccination programs and high vaccination uptake would benefit from such an extension. Little clinical data exist on the seropersistence of TBE antibodies after the 3rd dose of the primary immunization. In one study investigating TBE seropositivity 2 and 3 years after the third vaccination50 subjects aged 18-50 years showed higher seropositivity rates (88.7% and 92.3%, after 2 and 3 years, respectively) than those aged 51-67 years (65.5% and 70.9% after 2 and 3 years, respectively), thus confirming the appropriateness of the existing manufacturer recommendation for the administration of the first booster dose 3 years after completion of the primary series. There is no data on long-term seropersistence for the 2 Russian and the Chinese vaccines. Twelve months after primary immunization, seropositivity rates of 72%, 87%, and 77% were determined for EnceVir, TBE-Moscow, and the Chinese Vaccine, respectively.6
Most of the studies conducted in elderly individuals have shown consistently lower antibody concentrations compared with younger age groups.54-57 A cross-sectional study from the highly endemic Åland Islands found that age of the individual and number of vaccine doses were the 2 most important factors for determining the immune response to vaccination.50,55
The majority of these studies included subjects who received their primary vaccination series below the age of 50 years, which might have influenced the duration of seropositivity and B-cell memory.47,53 Unfortunately, few data exist on primary vaccination in individuals of more advanced age.
An observational study with FSME-IMMUN and Encepur administered to previously unvaccinated elderly subjects reported seropositivity rates of 95% and 80%, respectively, for subjects vaccinated with FSME-IMMUN (as measured by the Immunozym and Enzygnost ELISA Kits) and 65% and 80%, respectively, for subjects vaccinated with Encepur (as measured by the Immunozym and Enzygnost ELISA Kits).56
This study illustrates not only the reduced immune response after TBE vaccination seen in the elderly population, but it also gives evidence for dependence of serologic results on the commercial ELISA test systems. Unfortunately, this study was not evaluated using NT. One study, which compared the primary immune response in older and younger subjects, showed that subjects primed after the age of 50 years achieve not only lower titers but also experience a more rapid decline of neutralizing antibodies as compared to subjects primed at a younger age. Of note, almost no difference in the booster response was found between the 3 older age groups: 50–59 years, 60–69 years, and >69 years of age, indicating that responsiveness to vaccination is impaired already by the age of 50.54
A relatively recent study investigated the immune response to a conventional primary immunization schedule with FSME-IMMUN in previously unvaccinated subjects >70 years of age.58 Four weeks after the second and third vaccinations, 98.5% and 99.3% of subjects were seropositive (=10) by NT, even if GMTs were generally lower. Although antibody concentrations are lower in the elderly, booster doses have been shown to sufficiently increase the antibody levels, indicating an adequate immune memory response in the elderly population as well. Moreover, one study showed that the quality of antibodies as measured by antibody avidity were intact despite the lower antibody titers.59 The findings described above underscore the importance of adhering to the recommended schedules, including the 3-year booster intervals in subjects age 60 years and older. Moreover, in the region of Stockholm an additional dose of the primary schedule is recommended for subjects older than 60 years of age. Such an explicit recommendation how-ever does not exist in other countries and existing epidemiological data do not support this recommendation.
Cellular immunity
Until recently little was known about the cellular immune response after TBE vaccination. Immunization with inactivated TBE vaccine has been reported to induce primarily a CD4+ T-cell response with a very low induction of CD8+ cells.60,61 More recent investigations of TBE ‘low- responders’ after vaccination showed a positive correlation with humoral and cellular immune responses upon booster vaccination: high or low TBE titers were associated with sufficient or lack of Ag-specific T-cell proliferation, respectively.62
Research published in 2016 reported on the cellular immune response after a booster vaccination of FSME-IMMUN, administered by subcutaneous and intramuscular routes, revealing that interleukin-2 (IL-2), interferon (IFN) gamma, and interleukin-10 (IL-10) levels, produced upon antigen re-stimulation of peripheral blood mononuclear cells (PBMCs), were already elevated prior to vaccination.63 This observation is in line with the fact that all study subjects had received multiple TBE vaccinations in the past and therefore had high numbers of TBE-specific effector memory T cells. Quantification of different T-cell subpopulations (naïve, memory, and suppressor T cells) before and 1 week after booster vaccination showed a relative decrease in regulatory T cells after vaccination. This is most likely due to an effector T-cell expansion induced by the booster vaccination and not the result of a decrease in the total number of regulatory T cells.63
Moreover, the investigators observed an increase in the percentage of CD4+ T cells combined with a slight relative decrease of CD8+ T cells after intramuscular vaccination and a relative decrease of effector memory CD4+ T cells after subcutaneous vaccination. However, the observed changes in the CD4+ and CD8+ T-cell sub-populations were very small and had no influence on neutralizing antibody titers.63 Whereas all these data were obtained after TBE booster immunization in previously vaccinated individuals, data are lacking on the cellular immune response in the context of TBE primary vaccination.
Vaccine effectiveness
Austria is a highly endemic country for TBE with a very long history of TBE immunization. Vaccination coverage has increased steadily since the 1970s, when the first TBE vaccine – FSME-IMMUN – was initially licensed. According to an investigation of the field effectiveness of TBE vaccines in Austria during the years 2000–2006, 88% of the Austrian population had a history of TBE vaccination, and 58% were vaccinated according to the licensed schedule.35 For the above-mentioned period, when FSME-IMMUN comprised 90% to 95% of the TBE vaccines administered in Austria, an effectiveness of approximately 99% was calculated for regularly vaccinated persons, with no statistically significant difference between age groups.35 Not a single case of TBE was recorded within the first year after a documented history of 2 vaccinations, thus achieving a vaccine effectiveness of 100% after 2 vaccinations. A later investigation of vaccine effectiveness for the years 2000-201136 showed a slight decrease of vaccination coverage to 85% in 2011. Nevertheless, similarly high rates of effectiveness were seen: 98.7% and 96.3% for regularly vaccinated subjects under best- and worst-case assumptions, respectively, and 92.5% and 91.3% for irregularly vaccinated subjects under best- and worst-case scenarios, respectively. These findings highlight the importance of adhering to the recommended vaccination schedule, as there is a considerably higher risk of acquiring TBE in irregularly vaccinated subjects. As a result of the high vaccination uptake in Austria, an estimated 4,000 TBE cases and 20 deaths were prevented between 2000 and 2011.35,36 During the same time, neighboring countries including the Czech Republic and Slovenia, which are also highly endemic for TBE but with very low vaccination coverage (16% in 2009 and 12% in 2008, respectively),36,64 experienced an increase in disease incidence.
In the context of a mass immunization program that started in 1996 in the highly endemic region of Sverdlovsk in Russia, an impressive decrease in TBE incidence could be achieved – from 42.1/100,000 in 1996 to 9.7/100,000 in 2000 to 5.1/100,000 in 2006. The vaccines used were TBE- Moscow (market share 80%); EnceVir (market share 6%); FSME-IMMUN (market share 12%); and Encepur (market share 2%). Based on these data, an overall vaccine effectiveness of 62% and 89% was estimated for the years 2000 and 2006, respectively.31 Nevertheless, rare cases of TBE breakthrough disease, primarily in subjects older than 50 years of age, have been reported after primary TBE vaccination but not after booster immunization.65-68
No effectiveness data are available for the Chinese vaccine. There is only a single report, from the Center for Disease Control and Prevention, of the Hailar Railway, which showed that since the use of the current generation TBE vaccine, no TBE cases had been reported in 2009 and 2010.6 However, details of the vaccination program (vaccination schedule, type of surveillance, etc.) are largely unknown.
Vaccination failures
Vaccine failures have been reported only occasionally. A retrospective investigation of breakthrough cases over a period of 8 years was conducted in Sweden.65 During this period, 19 verified and 8 probable cases of TBE vaccine failures were reported. No accepted and plausible rationale exists to explain the immunological mechanisms leading to a vaccination failure. Therefore, it is not clear whether primary low-level responsiveness after regular TBE vaccination may be a risk factor for vaccine breakthrough. In contrast to unvaccinated subjects, most patients with breakthrough disease already had high antibody avidity and strong neutralizing antibodies in the first sample taken after hospitalization. When combined with an observed delayed immunoglobulin M (IgM) antibody response, and therefore presenting the features of an anamnestic response, this immune profile was obviously not sufficient to prevent the disease.68 In 2019 a second retrospective study99 on vaccine breakthroughs in Sweden was published and identified particularly i) older age (over 50 years of age) , ii) immunocompromising comorbidities and number of preceding vaccinations as key parameters for a higher risk of vaccine failures. The authors recommend for those persons, who start with their primary immunization series after the age of 50 an extra priming dose to reduce this risk. In addition, this study could for the first time define the probability of vaccine failures with 5% in a vaccinated population. While the Swedish study found there is an indication for more severe disease courses in older age, a retrospective study on clinical severity of vaccine breakthroughs from Germany,100 however, could not identify a higher risk of more severe clinical disease in these patients
Safety and tolerability
The currently available European TBE vaccines have a well-established safety record.8,33 Safety and tolerability have been investigated in a number of clinical studies conducted in children and adults. Broad experience also comes from the field, with extensive pharmacovigilance over many years. Over the past decades, TBE vaccine formulations have been refined, thereby significantly reducing reactogenicity. In contrast, little published data are available on the safety of the two Russian vaccines and almost no data are available on the Chinese vaccine.69 Frequently reported reactions after TBE vaccination basically do not differ from those occurring after vaccination with other aluminum-adjuvanted vaccines, e.g., local pain, redness, and swelling at the injection site, as well as headache, fatigue, malaise, muscle pain, joint pain, and fever.
Safety has been investigated in the context of many clinical studies with FSME-IMMUN, involving more than 13,800children and adults.9-11,13,14,50 All adverse reactions observed during clinical studies and relevant reports to the pharmacovigilance departments of the manufacturers are summarized in the Summary of Product Characteristics, Table 3. The most frequently reported reactions to the vaccination are local pain ( ≥ 1/10), headache, fatigue, malaise, myalgia, and arthralgia (1/100 and <1/10), whereas the frequency of fever was uncommon (≥ 1/1.000 and <1/100). Adverse reactions to vaccination seen in children are similar to those observed in adults. However, children more frequently experience fever, especially young children after the first vaccination. In addition, young children commonly react to vaccination with irritability, appetite loss, and disturbed sleep.
Similarly, at least 4 clinical trials have established the safety profile of Encepur in children and adults12,18,20,22 (Table 3). Similar to FSME-IMMUN, the most frequently reported reactions to vaccination with Encepur are local pain, malaise, myalgia, and headache (>10% of vaccinees), whereas local redness, swelling, flu-like symptoms, nausea, arthralgia, and fever (primarily after the first vaccination) were observed in 1–10% of the vaccinees.
As of 2002, two TBE pediatric vaccines, FSME-IMMUN Junior (Baxter) and Encepur Children (Novartis/GSK), were marketed and at that time a post-marketing sentinel study was carried out in Austria. The study was conducted by the Institute for Vaccine Safety of the Austrian Green Cross and included 500 selected pediatricians and general practitioners who generated data on more than 25,000 vaccinations (85% with FSME-IMMUN). A total of 107 adverse events (AEs) were reported, with 69 (64.5%) of these occurring in children below the age of 2 years; also, 75.8% of the AEs were reported in association with the first vaccination. Fever was reported in 63 cases; 45 of these cases were mild, 15 moderate, and 3 severe (fever >39.5° C).70
Data derived from spontaneous reporting to the pharmacovigilance departments of manufacturers of both vaccines (FSME-IMMUN, for the period between 2001 and 2009, and Encepur, for the period between 2002 and 2009) indicate comparable rates of serious AEs (1.57 per 100,000 doses administered).41 According to safety grading, as published in a WHO position paper in 2011, currently available TBE vaccines are not causally associated with serious adverse vaccine reactions.71 Finally, although the safety sections of the SMPCs for FSME IMMUN and Encepur show some differences, it can be concluded that both vaccines have a similar safety and reactogenicity profile.
According to the Russian National Regulatory Authority, both Russian vaccines – TBE-Moscow and EnceVir – are safe and well tolerated,33,41 and their manufacturing process fulfills WHO standards. However, no official documentation of quality control exists and no published data from large, controlled safety trials are available. Small-scale observational studies with TBE-Moscow and EnceVir have suggested a moderate reactogenicity profile with no significant differences between the 2 vaccines. Post-marketing surveillance data did not identify any serious AEs.26,32,72
A study in children between 7 and 17 years of age comparing TBE-Moscow vaccine and FSME-Immun (old formulation; adult dose used also for children) found that fever was reported more frequently with TBE-Moscow vaccine; however, the differences were not significant.4
A passive, post-marketing surveillance review of EnceVir did not reveal any serious AEs up to 2010.72 In 2010 and 2011, some lots of EnceVir were associated with a high incidence of fever and allergic reactions, particularly in children and adolescents. As a result, these lots were withdrawn from the market and the vaccine indication was restricted to adults above the age of 17 years.73 No published safety data are available for the Chinese TBE vaccine.
Table 3: Safety and Reactogenicity of FSME-IMMUN and Encepur (source: SMPCs)
| Probability | ≥1/10 | ≥1/100 <1/10 | ≥1/1000 <1/100 | ≥1/10.000 <1/1000 | Not known |
|---|---|---|---|---|---|
| FSME-Immun 1st vaccination: n=3512 2nd vaccination: n=3477 3rd vaccination: n=3277 | Local reaction at injection site: e.g., Injection- site pain | Headache, nausea, myalgia, arthralgia, malaise, fatigue. | Lymphadeno-pathy, vomiting, fever (only exceptionally >39°C), injection-site hemorrhage. | Acute allergic reactions, somnolence, diarrhea, abdominal pain, vertigo, local reaction at injection site: redness, swelling, induration, pruritus, paraesthesia, inflammation | Herpes Zoster (in pre-exposed individuals), aggravation of autoimmune disease, anaphylactic reaction, visual impairment, photophobia, eye pain, demyelinating disorders, meningismus, encephalitis, neuritis, neuralgia, tachycardia, tinnitus, dyspnea, urticaria, rash, pruritus, dermatitis, erythema, hyperhidrosis, back pain, joint swelling, neck pain, musculoskeletal stiffness, pain in extremity, gait disturbance, chills, flu-like symptoms, weakness, edema |
| Encepur (Pooled data from clinical studies and post-marketing surveillance) | Transient pain at injection site, general malaise, myalgia, headache | Redness, swelling at injection site, flu-like symptoms, fever ≥38°, nausea, arthralgia | Arthralgia and myalgia (neck), vomiting | Granuloma at injection site, diarrhea, arthralgia and myalgia in the neck region, lymphadenopathy, neuritis-like symptoms, systemic allergic reactions - like urticaria, dyspnea, bronchospasm, hypotension, transient thrombocytopenia | Extremely rare: Guillain-Barre Syndrome |
Passive Immunization and post-exposure prophylaxis
For many years, passive immunization as well as post-exposure prophylaxis with TBEV IgG preparations (immune globulin concentrate) was a state-of-the-art treatment following a tick bite in unvaccinated subjects in Europe and Russia. Administration of an immunoglobulin concentrate for passive immunization was expected to protect against disease. However, passive immunization was blamed for antibody-mediated enhancement (ADE) of TBE infection in children,74 like ADE phenomena in Dengue infections. In the late 1990s, the use of these immunoglobulins after tick exposure in a TBE-endemic area was discontinued even if the enhancement of TBEV infection could not be proven, neither in humans nor in a mouse model.75,76 In Russia, especially in the highly endemic regions, post-exposure prophylaxis with immunoglobulins continues to be common practice. Russian studies report that timely administration of specific immunoglobulin after a tick bite can prevent clinical disease in about 80% of cases. The recommended dose is 0.05 mL/kg body weight of TBE immunoglobulin, whereby the antibody titer should not be less than 1:80.77,78 However, investigations of the TBE-specific neutralizing antibody titers in IVIG (intravenous immunoglobulin) preparations from different geographic regions showed significantly lower TBEV neutralization titers in Russian-IVIG preparations compared with European IVIG preparations.78
Post-exposure prophylaxis with TBE vaccines in persons with a tick bite has to take into account the vaccination status and the incubation period of the disease. An accepted approach is summarized in Table 4.79
Table 4: Post-exposure prophylaxis according to vaccination status
| Vaccination history (written documentation) | Interval between last immunization and tick sting | Interval between tick sting and physicians visitb | Recommendation |
|---|---|---|---|
| Unvaccinated or unknown | Not applicable | <4 weeks | Wait until ≥4 weeks after sting, then initiate immunization series |
| 1 dose | ≤ 14 days | Not relevant | Wait until ≥4 weeks after sting, then administer 2nd dose |
| 15 days - 1 year | <48 hours | Administer 2nd dose immediately | |
| ≥48 h | Wait until ≥4 weeks after sting, then administer 2nd dosea | ||
| ≥1 year | <48 h | Administer 2nd dose immediatelya | |
| ≥ 48 h | Wait until ≥4 weeks after sting, then administer 2nd dosea | ||
| ≥2 | Additional vaccination according to regular schedule |
aTesting of antibody response recommended. If not possible, count this vaccination as the first one in basic immunization schedule;
bIf time elapsed is not to be determined, use schedule: >48 h after tick bite
TBE vaccination in special patient groups
Underlying medical conditions can influence the outcome of vaccination by reducing the immune response. Alternatively, vaccination can theoretically cause a deterioration or exacerbation of the underlying condition. Therefore, the decision to vaccinate or not in subjects with serious medical conditions must be based on a careful risk/benefit analysis. Several studies have investigated immune response effects or influence on the course of the disease in the context of TBE immunization. A controlled trial on TBE vaccination in patients with multiple sclerosis found no association between the vaccination and disease activity (as detected by magnetic resonance imaging [MRI]), clinical relapse, or disease progression.80
Another study investigated the effect of TBE vaccination in medically immunosuppressed patients with rheumatoid arthritis.81 The patients (N=66) received a TBE primary immunization series while they were on regular treatment with a tumor necrosis factor inhibitor (TNFi) and/or methotrexate (MTX) for at least 1 year. One month after the third dose, 39% (26/66) of the patients and 79% (44/56) of the healthy controls had seroprotective NT levels. The relatively low SPR observed in the control group may be attributed to the fact that 37 and 35 of the patients and controls, respectively, were 60 years of age and older. Interestingly, the group of patients receiving a combined treatment (TNFi + MTX) had a significantly lower protection rate compared with healthy controls (36% vs 87%), while rates in patients treated with only a single medication did not differ from those seen in healthy controls. The significant difference in SPR remained even when an additional priming dose was given to all patients and healthy controls who were ≥ 60 years old: 31% (9/29) in the patient group compared with 81% (17/21) in the control group. In addition, this study demonstrated that in older patients (>60 years of age) immunosenescence apparently added to the treatment effects, leading to seroconversion rates of only around 30% after 4 doses of TBE vaccine in patients with combined immuno-suppressive treatments.
The effect of TBE vaccination using an abbreviated immunization schedule was also compared in 31 heart transplant recipients, under cyclosporine-based immunosuppression, and 29 controls.82 Immune response (seroconversion rates [SCRs] and GMTs) were markedly reduced in the transplant recipients as compared with the control group. Even though the vaccine used in this study is no longer on the market (previous generation of Encepur, stabilized with polygeline), the findings are consistent with more recent investigations.
Public health considerations
While no formal vaccine efficacy study has been conducted with any TBE vaccine, effectiveness and pharmacoeconomic studies have been conducted, and the evidence for the public health impact of TBE immunization is indisputable. The most impressive example can be obtained from Austria, a country with a longstanding tradition of TBE immunization and reliable epidemiological data since the early 1970s. Since that time, vaccination coverage has increased steadily with currently 85% to 88% of the population having received at least 1 dose of TBE vaccine.36 As a result, disease incidence dropped from approximately 700 to fewer than 100 cases per year, while in neighboring countries, with low vaccine coverage, the disease incidence has increased (see chapter on epidemiology).
Little information is published on the economic burden of TBE disease. Based on the finding that the Austrian TBE vaccination campaigns for the period 1981–1990 led to a reduction of more than 50% of clinical TBE cases, a benefit of €24 million was calculated versus the pre-vaccination era. Using a linear trend prognostic model for the further decline of TBE cases while vaccination coverage reached 85% by 2000, the author concluded that for the period 1991 to 2000, a total cost saving of €60 million can be estimated.83 Epidemiological trends and progress in vaccination coverage have confirmed these assumptions.36 The majority of endemic countries in Europe, as well as Russia, have TBE vaccination recommendations in place, targeting primarily at-risk groups. More recently, recommendations for travelers to endemic regions were issued in many countries (see Chapter 12b). As TBE disease was believed to be less severe in children, some countries had recommendations for adults only. More recent publications on severe disease courses and underestimation of long-term sequelae in children have led to adaptations of the vaccination recommendations for children in some countries. For instance, in Sweden, the age cut-off was reduced in 2012 from 7 years to 3 years of age and in 2013 from 3 years to 1 year of age.
In 2011, the WHO published a position paper on TBE vaccination33 recommending vaccination of all age groups in areas of high pre-vaccination disease incidence, defined as an incidence of ≥ 5/100,000 population per year, while in regions with lower incidence, vaccination recommendations should be confined to groups of the population exposed to a particular risk. Furthermore, the WHO also recommends vaccination of travelers planning outdoor activities in endemic areas during the active tick season.84 In 2012, TBE became notifiable on the European level at the European Centre for Disease Prevention and Control (ECDC), which is a further, important step towards comprehensive and continuous assessment of the disease epidemiology across Europe.
Recently a cost/benefit analysis has became available. In Sweden, where the area of Stockholm is highly endemic and the number of cases is increasing despite the increased uptake of TBE vaccines, earlier studies showed that low-income households have lower vaccination coverage even when they are at high risk. The newly performed analysis showed a gain in cost per QALY (Quality-adjusted Life Years) of a free vaccinations program for the Stockholm county, especially for children of 3 years old, below generally acceptable cost-effectiveness thresholds in Sweden.96
Contact:
eva.poellabauer@meduniwien.ac.at
Citation:
Pöllabauer EM, Kollaritsch H. Prevention: Vaccines and Immunoglubins. Chapter 14. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi: 10.33442/26613980_14-6
References
- Klockmann U, Bock HL, Franke V, Hein B, Reiner G, Hilfenhaus J. Preclinical investigations of the safety, immunogenicity and efficacy of a purified, inactivated tick-borne encephalitis vaccine. J Biol Stand. 1989;17:331-42.
- Girgsdies OE, Rosenkranz G. Tick-borne encephalitis: development of a paediatric vaccine. A controlled, randomized, double-blind and multicentre study. Vaccine. 1996;14:1421-8.
- Vorob’eva MS, Rasshchepkina MN, Ladyzhenskaia IP. [Vaccines, immunoglobulins, and test systems for the prevention and diagnosis of tick-borne encephalitis]. Vopr Virusol. 2007;52:30-6.
- Pavlova LI, Gorbunov MA, Vorob’eva MS, et al. [A cultured concentrated inactivated vaccine against tick-borne encephalitis studied during the immunization of children and adolescents]. Zh Mikrobiol Epidemiol Immunobiol. 1999:50-3
- Lu Z, Bröker M, Liang G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. 2008;8:713-20.
- Xing Y, Schmitt HJ, Arguedas A, Yang J. Tick-borne encephalitis in China: A review of epidemiology and vaccines. Vaccine. 2017;35:1227-37.
- Schuller E, Klade CS, Heinz FX, et al. Effect of pre-existing anti-tick-borne encephalitis virus immunity on neutralising antibody response to the Vero cell-derived, inactivated Japanese encephalitis virus vaccine candidate IC51. 2008;26:6151-6.
- Demicheli V, Debalini MG, Rivetti A. Vaccines for preventing tick-borne encephalitis. Cochrane Database Syst Rev.2009:CD000977.
- Loew-Baselli A, Poellabauer EM, Pavlova BG, et al. Prevention of tick-borne encephalitis by FSME-IMMUN® vaccines: review of a clinical development programme. Vaccine. 2011;29:7307- 19.
- Ehrlich HJ, Pavlova BG, Fritsch S, et al. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: evaluation of safety and immunogenicity. Vaccine. 2003;22:217-23.
- Loew-Baselli A, Konior R, Pavlova BG, et al. Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: results of two large phase 3 clinical studies. 2006;24:5256-63.
- Schöndorf I, Beran J, Cizkova D, Lesna V, Banzhoff A, Zent O. Tick-borne encephalitis (TBE) vaccination: applying the most suitable vaccination schedule. Vaccine. 2007;25:1470-5.
- Pöllabauer EM, Fritsch S, Pavlova BG, et al. Clinical evaluation to determine the appropriate paediatric formulation of a tick- borne encephalitis vaccine. Vaccine. 2010;28:4558-65.
- Pöllabauer EM, Pavlova BG, Löw-Baselli A, et al. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine. 2010;28:4680-5.
- Prymula R, Pöllabauer EM, Pavlova BG, et al. Antibody persistence after two vaccinations with either FSME-IMMUN® Junior or ENCEPUR® Children followed by third vaccination with FSME-IMMUN® Junior. Hum Vaccin Immunother. 2012;8:736-42
- Wittermann C, Petri E, Zent O. Long-term persistence of tick-borne encephalitis antibodies in children 5 years after first booster vaccination with Encepur Children. 2009;27:1585-8.
- Zent O, Bröker M. Tick-borne encephalitis vaccines: past and present. Expert Rev Vaccines. 2005;4:747-55.
- Zent O, Beran J, Jilg W, Mach T, Banzhoff A. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine. 2003;21:738-41.
- Zent O, Jilg W, Plentz A, et al. Kinetics of the immune response after primary and booster immunization against tick-borne encephalitis (TBE) in adults using the rapid immunization schedule. 2003;21:4655-60.
- Zent O, Hennig R, Banzhoff A, Bröker M. Protection against tick-borne encephalitis with a new vaccine formulation free of protein-derived stabilizers. J Travel Med. 2005;12:85-93.
- Schoendorf I, Ternak G, Oroszlàn G, Nicolay U, Banzhoff A, Zent O. Tick-borne encephalitis (TBE) vaccination in children: advantage of the rapid immunization schedule (i.e., days 0, 7, 21). Hum Vaccin. 2007;3:42-7.
- Zent O, Banzhoff A, Hilbert AK, Meriste S, Sluzewski W, Wittermann C. Safety, immunogenicity and tolerability of a new pediatric tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. 2003;21:3584-92.
- Wittermann C, Schöndorf I, Gniel D. Antibody response following administration of two paediatric tick-borne encephalitis vaccines using two different vaccination schedules. Vaccine. 2009;27:1661-6.
- Leonova GN, Ternovoi VA, Pavlenko EV, Maistrovskaya OS, Protopopova EV, Loktev VB. Evaluation of vaccine Encepur Adult for induction of human neutralizing antibodies against recent Far Eastern subtype strains of tick-borne encephalitis virus. Vaccine. 2007;25:895-901.
- Chiba N, Osada M, Komoro K, Mizutani T, Kariwa H, Takashima I. Protection against tick-borne encephalitis virus isolated in Japan by active and passive immunization. Vaccine. 1999;17:1532-9
- Krasilnikov I, Mischenko IA, Sharova OI, Vorob’eva M. Development of technology of tick-borne encephalitis vaccine (strain 205). Int J Med Microbiol. 2002;291:173.
- Gorbunov MA, Pavlova LI, Vorob’eva MS, Raschepkina MN, Stronin OB. Results of clinical evaluation of EncoVir vaccine against tick-borne encephalitis. Epidem Vaccinoprophil. 2002;5:49.
- Krasilnikov IV, Mischenko IA, Sharova OI, et al. Vaccine “EnceVir”: development and implementation in practical use. Biopreparations. 2004;2:21-4.
- Pavlova LI, Stavitskaya IV, Gorbunov MA, al e. Immunizations of children and adolescents with concentrated purified vaccines against TBE. 2003;1:24-8.
- Stavitskaya IV, Shkuratova OV, Pavlova LI, Shutova NA, Sharova OI. Immunological effectiveness of EnceVir in children.Biopreparations. 2004;2:34–6.
- Romanenko VV, Esiunina MS, Kiliachina AS. [Experience in implementing the mass immunization program against tick-borne encephalitis in the Sverdlovsk Region]. Vopr Virusol. 2007;52:22-5.
- Borodina TN, Evtoushok GA, Tevelenok OG, Opeikina NN. Epidemiological effectiveness of vaccination against TBE in Krasnoyarsk region. Biopreparations. 2004;2:30-1.
- Vaccines against tick-borne encephalitis (TBE): WHO position paper, 10 June, 2011. Wkly Epidemiol Rec.2011;86:241-56.
- Leonova GN, Pavlenko EV. Characterization of neutralizing antibodies to Far Eastern tick-borne encephalitis virus subtype and the antibody avidity for four tick-borne encephalitis vaccines in humans. Vaccine. 2009;27:2899-904.
- Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25:7559-67.
- Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis.2013;19:69-76.
- Schosser R, Reichert A, Mansmann U, Unger B, Heininger U, Kaiser R. Irregular tick-borne encephalitis vaccination schedules: the effect of a single catch-up vaccination with FSME-IMMUN. A prospective non-interventional Vaccine. 2014;32:2375-81.
- Rendi-Wagner P, Kundi M, Zent O, et al. Immunogenicity and safety of a booster vaccination against tick-borne encephalitis more than 3 years following the last immunisation. Vaccine. 2004;23:427-34.
- Jarmer J, Zlatkovic J, Tsouchnikas G, et al. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol. 2014;88:13845-57.
- Holzmann H, Kundi M, Stiasny K, et al. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol. 1996;48:102-7.
- Kollaritsch H, Krasilnikov V, Holzmann H, et al. Background Paper on Vaccines and Vaccination Against Tick-borne Encephalitis (TBE). Geneva, WHO Strategic Advisory Group of Experts on Immunization 2011.
- Holzmann H, Vorobyova MS, Ladyzhenskaya IP, et al. Molecular epidemiology of tick-borne encephalitis virus: cross-protection between European and Far Eastern subtypes. 1992;10:345-9.
- Orlinger KK, Hofmeister Y, Fritz R, et al. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis. 2011;203:1556-64.
- Domnich A, Panatto D, Arbuzova EK, et al. Immunogenicity against Far Eastern and Siberian subtypes of tick-borne encephalitis (TBE) virus elicited by the currently available vaccines based on the European subtype: systematic review and meta-analysis. Hum Vaccin Immunother. 2014;10:2819- 33.
- Beck Y, Fritz R, Orlinger K, et al. Molecular Basis of the Divergent Immunogenicity of Two Pediatric Tick-Borne Encephalitis Virus Vaccines. J Virol. 2015;90:1964-72.
- Rendi-Wagner P, Zent O, Jilg W, Plentz A, Beran J, Kollaritsch H. Persistence of antibodies after vaccination against tick-borne encephalitis. Int J Med Microbiol. 2006;296 Suppl 40:202-7.
- Paulke-Korinek M, Kundi M, Laaber B, et al. Factors associated with seroimmunity against tick-borne encephalitis virus 10 years after booster vaccination. 2013;31:1293-7.
- Paulke-Korinek M, Rendi-Wagner P, Kundi M, Laaber B, Wiedermann U, Kollaritsch H. Booster vaccinations against tick-borne encephalitis: 6 years follow-up indicates long-term protection. Vaccine. 2009;27:7027-30.
- Zent O, Plentz A, Schwarz TF, et al. TBE booster immunization according to the rapid immunization schedule: are 3-year booster intervals really necessary? 2004;23:312-5.
- Loew-Baselli A, Poellabauer EM, Pavlova BG, et al. Seropersistence of tick-borne encephalitis antibodies, safety and booster response to FSME-IMMUN 0.5 ml in adults aged 18-67 years. Hum Vaccin. 2009;5:551-6.
- Rendi-Wagner P, Kundi M, Zent O, et al. Persistence of protective immunity following vaccination against tick-borne encephalitis—longer than expected? 2004;22:2743- 9.
- Rendi-Wagner P, Paulke-Korinek M, Kundi M, Wiedermann U, Laaber B, Kollaritsch H. Antibody persistence following booster vaccination against tick-borne encephalitis: 3-year post-booster follow-up. 2007;25:5097-101.
- Beran J, Xie F, Zent O. Five year follow-up after a first booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates long-term antibody persistence and safety. Vaccine. 2014;32:4275-80.
- Weinberger B, Keller M, Fischer KH, et al. Decreased antibody titers and booster responses in tick-borne encephalitis vaccinees aged 50-90 years. Vaccine. 2010;28:3511-5.
- Lindblom P, Wilhelmsson P, Fryland L, et al. Factors determining immunological response to vaccination against tick-borne encephalitis virus in older individuals. PLoS One. 2014;9:e100860.
- Jilkova E, Vejvalková P, Stiborová I, Skorkovský J, Král V. Serological response to tick-borne encephalitis (TBE) vaccination in the elderly—results from an observational study. Exp Opin Biol Ther. 2009;9:797-803.
- Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck- Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. 2005;23:3232-5.
- Wanke K, von Braun A, Häberli L, et al. Immunogenicity and safety of tick-borne encephalitis vaccination in healthy elderly individuals. Paper presented at: ECCMID, 2012; London, UK.
- Stiasny K, Aberle JH, Keller M, Grubeck-Loebenstein B, Heinz FX. Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis. PLoS One. 2012;7:e34145.
- Aberle JH, Aberle SW, Kofler RM, Mandl CW. Humoral and cellular immune response to RNA immunization with flavivirus replicons derived from tick-borne encephalitis virus. J Virol. 2005;79:15107-13.
- Gomez I, Marx F, Saurwein-Teissl M, Gould EA, Grubeck- Loebenstein B. Characterization of tick-borne encephalitis virus-specific human T lymphocyte responses by stimulation with structural TBEV proteins expressed in a recombinant baculovirus. Viral Immunol. 2003;16:407-14.
- Garner-Spitzer E, Wagner A, Paulke-Korinek M, et al. Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol. 2013;191:2426-36.
- Hopf S, Garner-Spitzer E, Hofer M, Kundi M, Wiedermann U. Comparable immune responsiveness but increased reactogenicity after subcutaneous versus intramuscular administration of tick-borne encephalitis (TBE) vaccine. Vaccine. 2016;34:2027-34.
- Stefanoff P, Polkowska A, Giambi C, et al. Reliable surveillance of tick-borne encephalitis in European countries is necessary to improve the quality of vaccine recommendations. Vaccine. 2011;29:1283-8.
- Andersson CR, Vene S, Insulander M, Lindquist L, Lundkvist A, Gunther G. Vaccine failures after active immunization against tick-borne encephalitis. 2010;28:2827-31.
- Koppi S, Faé P, Hartmann G, Höftberger R, Holzmann H. [Fatal outcome of tick-borne encephalitis despite complete active vaccination]. 2011;82:506, 8.
- Plisek S, Honegr K, Beran J. TBE infection in an incomplete immunized person at-risk who lives in a high-endemic area—impact on current recommendations for immunization of high-risk groups. Vaccine. 2008;26:301-4.
- Stiasny K, Holzmann H, Heinz FX. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine. 2009;27:7021-6.
- Kollaritsch H, Paulke-Korinek M, Holzmann H, Hombach J, Bjorvatn B, Barrett A. Vaccines and vaccination against tick-borne encephalitis. Exp Rev Vacc 2012;11:1103-19.
- Weinzettel R, Ertl S, Zwiauer K. [FSME monitoring: monitoring of adverse events of tick-borne-encephalitis vaccines by selected paediatricians and general practitioners]. Wien Med Wochenschr. 2007;157:107-10.
- Vaccines against tick-borne encephalitis (TBE): WHO position paper, 10 June, 2011 [Grading safety]. 2011; available at: http://www.who.int/immunization/TBE_grad_safety.pdf? ua=1.
- Il’chenko TE, Bilalova GP, Stavitskaya NX, Solanik RG, Bistritskaya LD, Krasnilikov IV. Organziation of Public Health. Siberian J Med. 2009;2:50-5.
- Vaccines against tick-borne encephalitis (TBE): WHO position paper, 10 June, 2011 [Grading crossprotection]. 2011; available at: http://www.who.int/immunization/TBE_grad_crossprotection.pdf?ua=1.
- Kluger G, Schöttler A, Waldvogel K, et al. Tickborne encephalitis despite specific immunoglobulin prophylaxis. Lancet. 1995;346:1502.
- Kreil TR, Maier E, Fraiss S, Eibl MM. Neutralizing antibodies protect against lethal flavivirus challenge but allow for the development of active humoral immunity to a nonstructural virus protein. J Virol. 1998;72:3076-81.
- Brӧker M, Kollaritsch H. After a tick bite in a tick-borne encephalitis virus endemic area: current positions about post-exposure treatment. Vaccine. 2008;26:863-8.
- Pen’evskaia N, Rudakov N. [Efficiency of use of immunoglobulin preparations for the postexposure prevention of tick-borne encephalitis in Russia (a review of semi-centennial experience)]. Med Parazitol (Mosk). 2010;1:53-9.
- Rabel PO, Planitzer CB, Farcet MR, Kreil TR. Tick-borne encephalitis virus-neutralizing antibodies in different immunoglobulin preparations. Clin Vaccine Immunol. 2012;19:623-5.
- Impfplan Österreich 2017. Vienna, Austria: Ministerium Frauen Gesundheit;2017.
- Baumhackl U, Franta C, Retzl J, Salomonowitz E, Eder G. A controlled trial of tick-borne encephalitis vaccination in patients with multiple sclerosis. 2003;21 Suppl 1:S56- 61.
- Hertzell KB, Pauksens K, Rombo L, Knight A, Vene S, Askling HH. Tick-borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: A prospective, open-label, multi-centre study. 2016;34:650-5.
- Dengler TJ, Zimmermann R, Meyer J, Sack FU, Girgsdies O, Kübler WE. Vaccination against tick-borne encephalitis under therapeutic immunosuppression. Reduced efficacy in heart transplant recipients. Vaccine. 1999;17:867-74.
- Schwarz B. [Health economics of early summer meningoencephalitis in Austria. Effects of a vaccination campaign 1981 to 1990]. Wien Med Wochenschr. 1993;143:551-5.
- International Travel and Health, chapter 6. 2010; available at: http://www.who.int/ith/ITH2010chapter6.pdf.
- Zavadska D, Anca I, André F, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother. 2013;9:362-74.
- ECDC, European Center for Disease Prevention and Control. Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. 2012; available at: http://ecdc.europa.eu/en/ publications/Publications/TBE-in-EU-EFTA.pdf.
- Konior R, Brzostek J, Poellabauer EM, Jiang Q, Harper L, Erber W. Seropersistence of TBE virus antibodies 10 years after first booster vaccination and response to a second booster vaccination with FSME-IMMUN 0.5mL in adults. Vaccine. 2017 Jun 16;35(28):3607-3613
- Beran J, Lattanzi M, Xie F, Moraschini L, Galgani I. Second five-year follow-up after a booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates at least 10 years antibody Vaccine. 2018 Feb 1
- Steffen, NECTM, 2018
- Bröker M, Eickmann M, Stadler K. Genetic Stability of a Tick-Borne Encephalitis (TBE) Virus Strain used for the Production of a TBE Vaccine. J Vaccin Vaccin 2011;02(01).
- Xing Yi, Schmitt Heinz-Josef, Arguedas Adriano, Yang Junfeng. Tick-borne encephalitis in China: A review of epidemiology and vaccines. 2017;35:1227–1237
- Bröker M, Schöndorf I. Are tick-borne encephalitis vaccines interchangeable? Exp Rev Vaccines. 2006;5(4):461-466
- Litzba N, Zelená H, Kreil TR, Niklasson B, Kühlmann-Rabens I, Remoli ME, Niedrig M. Evaluation of different serological diagnostic methods for tick-borne encephalitis virus: enzyme-linked immunosorbent, immunofluorescence, and neutralization assay. Vector Borne Zoonotic Dis. 2014;14(2):149-59. doi: 10.1089/vbz.2012.1287. Epub 2013 Dec 20.
- Leonova GN, Pavlenko EV. Characterization of neutralizing antibodies to far Eastern tick-borne encephalitis virus subtype and the antibody avidity for four tick-borne encephalitis vaccines in humans. Vaccine. 2009;27(21):2899-2904.
- Maikova GB, Chernokhaeva LL, Rogova YV, et al. Ability of inactivated vaccines based on far-eastern tick-borne encephalitis virus strains to induce humoral immune response in originally seropositive and seronegative recipients. J Med Virol. 2019;91(2):190-200.
- Shedrawy J, Henriksson M, Hergens MP, Askling HH. Estimating costs and health outcomes of publicly funded tick-borne encephalitis vaccination: A cost-effectiveness analysis. 2018;36(50):7659-7665.
- Bundesamt für Gesundheit (BAG) Bulletin 6/2019, 4 Feb., 2019
- Poellabauer E, Angermayr R, Behre U, Zhang P, Harper L, Schmitt HJ, Erber W. Seropersistence and booster response following vaccination with FSME-IMMUN in children, adolescents, and young adults. Vaccine. 2019 May 27;37(24):3241-3250.
- Hansson KE, Rosdahl A, Insulander M, Vene S, Lindquist L, Gredmark-Russ S, Askling HH. Tick-borne Encephalitis Vaccine Failures: A 10-year Retrospective Study Supporting the Rationale for Adding an Extra Priming Dose in Individuals Starting at Age 50 Years. Clin Infect Dis. 2020 Jan 2;70(2):245-251
- Dobler G, Kaier K, Hehn P, Böhmer MM, Kreusch TM, Borde JP..Tick-borne encephalitis virus vaccination breakthrough infections in Germany – A retrospective analysis from 2001-2018. Clin Microbiol Infect. 2019 Dec 13. pii: S1198-743X(19)30653-6
- Bradt V, Malafa S, von Braun A, Jarmer J, Tsouchnikas G, Medits I, Wanke K, Karrer U, Stiasny K, Heinz FX. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. NPJ Vaccines. 2019 Sep 6;4:38.
- Vorovitch MF,Maikova GB, Chernokhaeva LL, Romanenko VV, Karganova GG, Ishmukhametov AA. Comparison of the Immunogenicity and Safety of Two Pediatric TBE Vaccines Based on the Far Eastern and European Virus Subtypes. Adv Virol. 2019 Dec 24;2019:5323428