Lidia Chitimia-Dobler, Ute Mackenstedt and Olaf Kahl
Key Points
- The natural cycle of the TBE virus is dependent on vector ticks and reservoir hosts.
- There are differing transmission cycles in varying environments, from cold northern coniferous forests to temperate central European forests.
- Within a natural transmission cycle, there are different ways of transmission: tick-to-tick (transovarial, sexual), host-to-tick (viremic), and also tick-to-tick and host-to-host.
- The complexity of natural transmission cycles is inadequately explored and poorly understood.
Introduction
Ticks play a critical role in the transmission of a wide variety of viral, bacterial, and protozoan pathogens to humans and animals.1,2 In the case of humans, infection is accidental as these transmission cycles are invariably enzootic with the natural hosts most frequently being wild birds and mammals.1 In order to be tangentially affected by such cycles, humans must be bitten by a vector tick species found in habitats visited by humans, as well as the tick’s usual hosts, as the dispersal of ticks not attached to hosts covers only very short distances.3 In addition, the tick has to accept humans as a suitable host, meaning that the species involved usually have a broad host spectrum.
Nevertheless, these tick species may only be part of the transmission cycle, with eco-epidemiologically significant sub-cycles involving tick species not commonly in contact with humans.4,5 Thus, the transmission of tick-borne pathogens often comprises a complex network of interactions involving several tick and host species. Below, we provide background to the biology of ticks and how this can influence, specifically, the eco-epidemiological cycle of TBEV.
Structure and morphology
Ticks are a group of hematophagous ectoparasites with about 910 living species.6 They belong to the phylum Arthropoda, the class Arachnida, the superorder Acarina, and the order Ixodida, and they are exclusively parasitic. The Ixodida contain 3 families: the Ixodidae with 14 genera (hard ticks), the Argasidae with genera (soft ticks), and the Nuttalliellidae, represented by only one species, Nuttalliella namaqua.7,8,9 All the tick species involved in the eco-epidemiological cycle of TBEV belong to the Ixodidae. Details of tick biology generally can be found in a variety of publications, for example in Nicholson et al.,8 Petney et al.,10 and Sonenshine and Roe,11 and a list of valid species names in Guglielmone and Nava.12 The following genera of ticks contain species known to transmit TBEV.
Ixodes is the largest tick genus, with 244 described species worldwide.7 Ixodes species are characterized by a distinct groove that encircles the anus anteriorly and a lack of eyes. Males have 7 sclerotized ventral plates that are absent in the males of other genera. The genus Ixodes has been subdivided in roughly 15 subgenera (e.g. Ixodes, Pholeoixodes) on the basis of morphology.13,14 The genus has a worldwide distribution, including parts of Antarctica.8,15 Some species are particularly important as vectors of TBEV: Ixodes ricinus the ‘castor bean tick’ or ‘sheep tick’ in Europe, Ixodes persulcatus ‘the taiga tick’ in northeastern Europe and northern Asia, and Ixodes ovatus in the forest belt of middle Asia and Japan.
The genus Dermacentor has 35 species worldwide.7 The basis capituli appears rectangular when viewed dorsally. A pair of medially directed spurs occurs on the first pair of coxae. The palps are short and thick. The scutum is almost always ornamented. Dermacentor species are found mostly in Europe, Asia, and North America.15 In Europe, TBEV has been recovered from 2 species, Dermacentor reticulatus (‘the ornate dog tick’), Dermacentor marginatus (‘the ornate sheep tick’), and in Asia from Dermacentor nuttalli. Haemaphysalis is the second largest tick genus.7 This eyeless genus can, in most cases, be identified by a pronounced lateral projection of palpal segment 2, which extends well beyond the basis capituli. In Europe, TBEV has been recovered from Haemaphysalis punctata (‘the red sheep tick’), Haemaphysalis concinna in Europe and Asia, and from Haemaphysalis longicornis in Asia.8,15
The genus Hyalomma is relatively small with 27 species of small- to large-sized ticks.16 They are characterized by their elongated palps, which are at least twice as long as wide. The distinct eyes are located in sockets adjacent to the postero-lateral edges of the scutum that is unornamented. The distribution of Hyalomma species is limited to the Old World, primarily to arid or semiarid habitats. Hyalomma marginatum (‘the Mediterranean Hyalomma’) is the only member of this genus from which TBEV has been recovered.
The biology of ticks
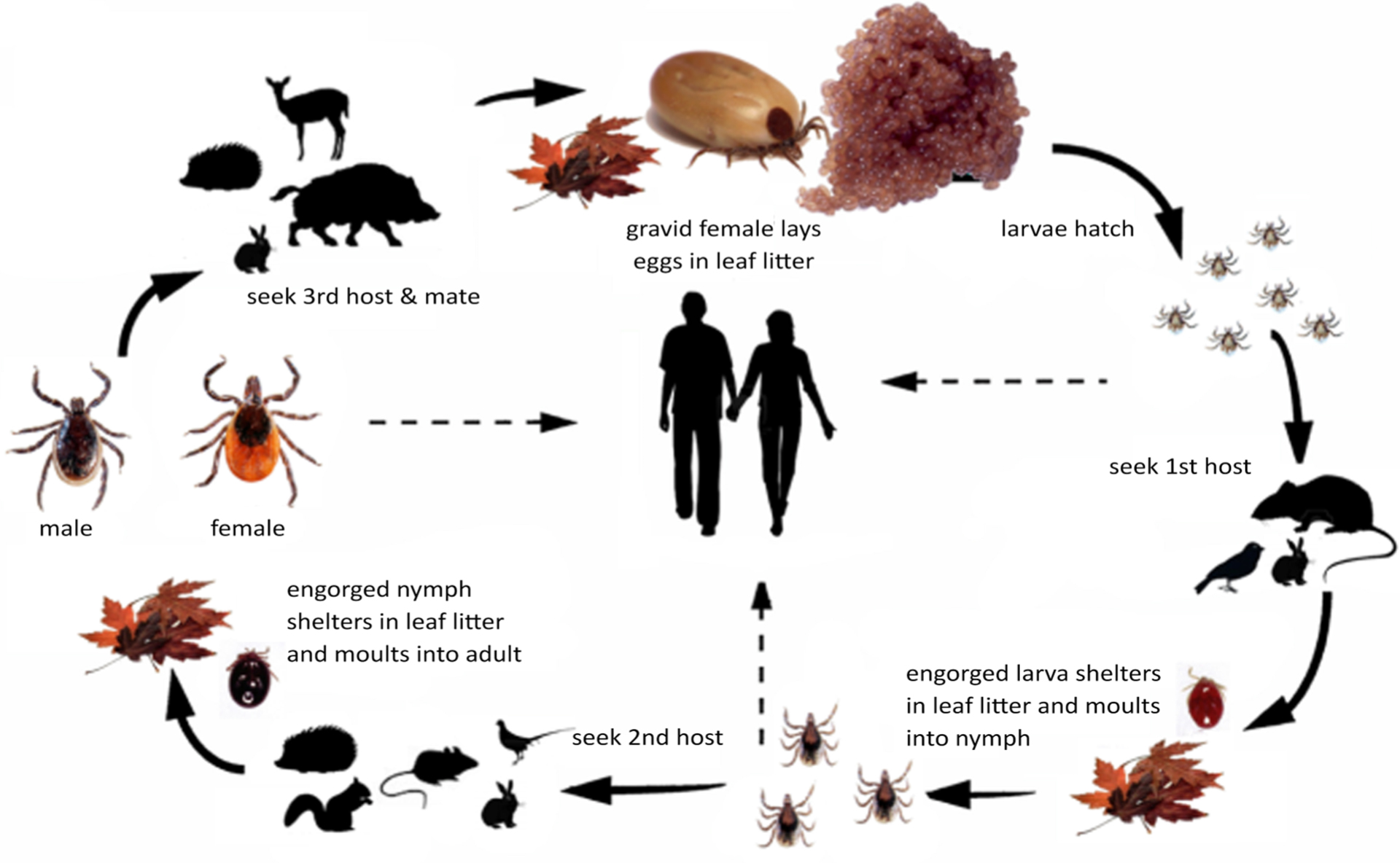
All the species known to transmit TBEV have a 3-host life cycle (Figure 1). Each postembryonic life stage requires a blood meal from a suitable host, after which the tick detaches and molts in the leaf litter. The arrows with broken lines in the figure show the potential transmission paths to humans. The line from larvae to humans indicates that transovarial trans-mission from an infected female can happen which results in infective larvae. Infection of the tick can occur when larvae, nymphs, or females feed on an infective host (see below).
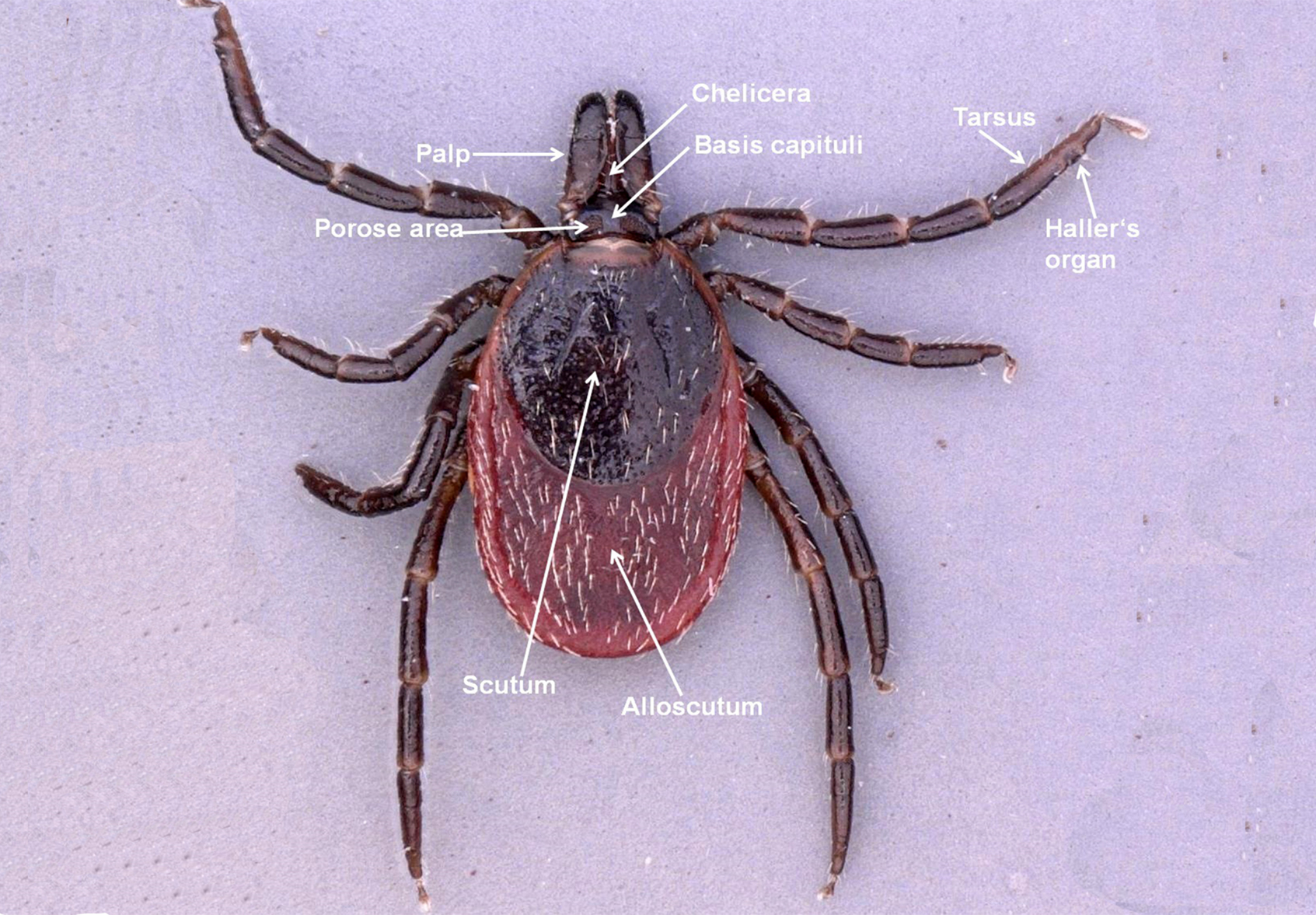
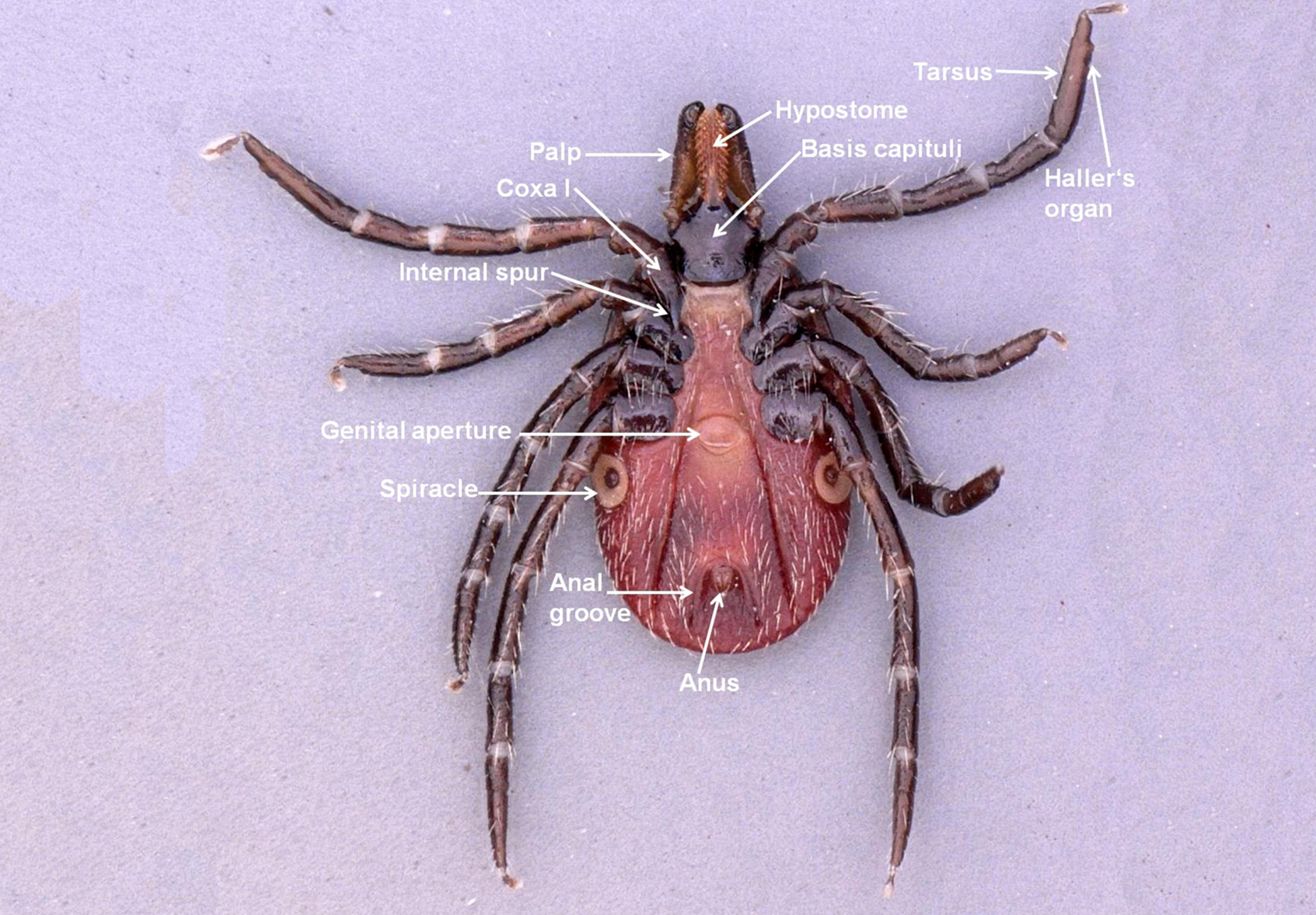
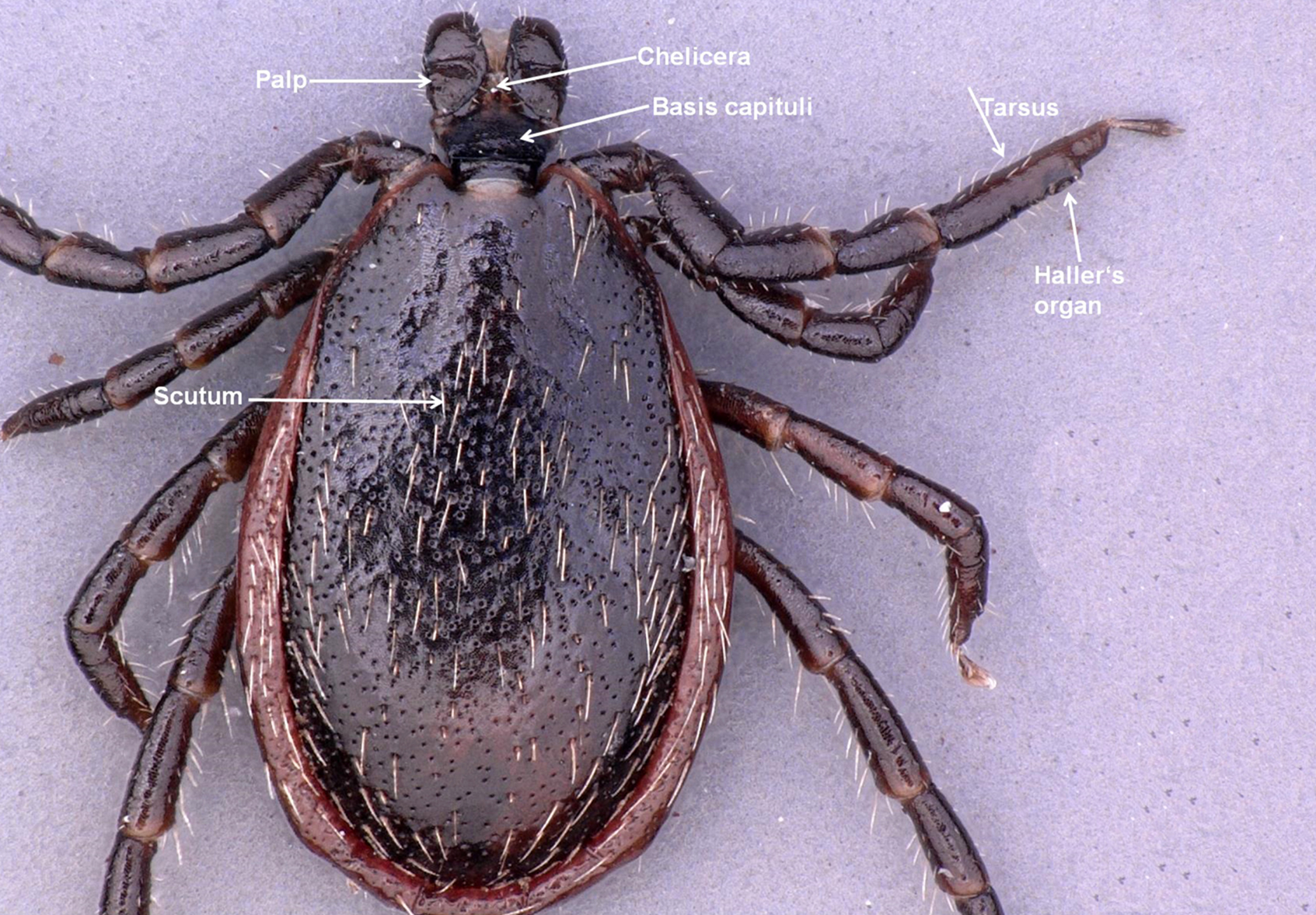
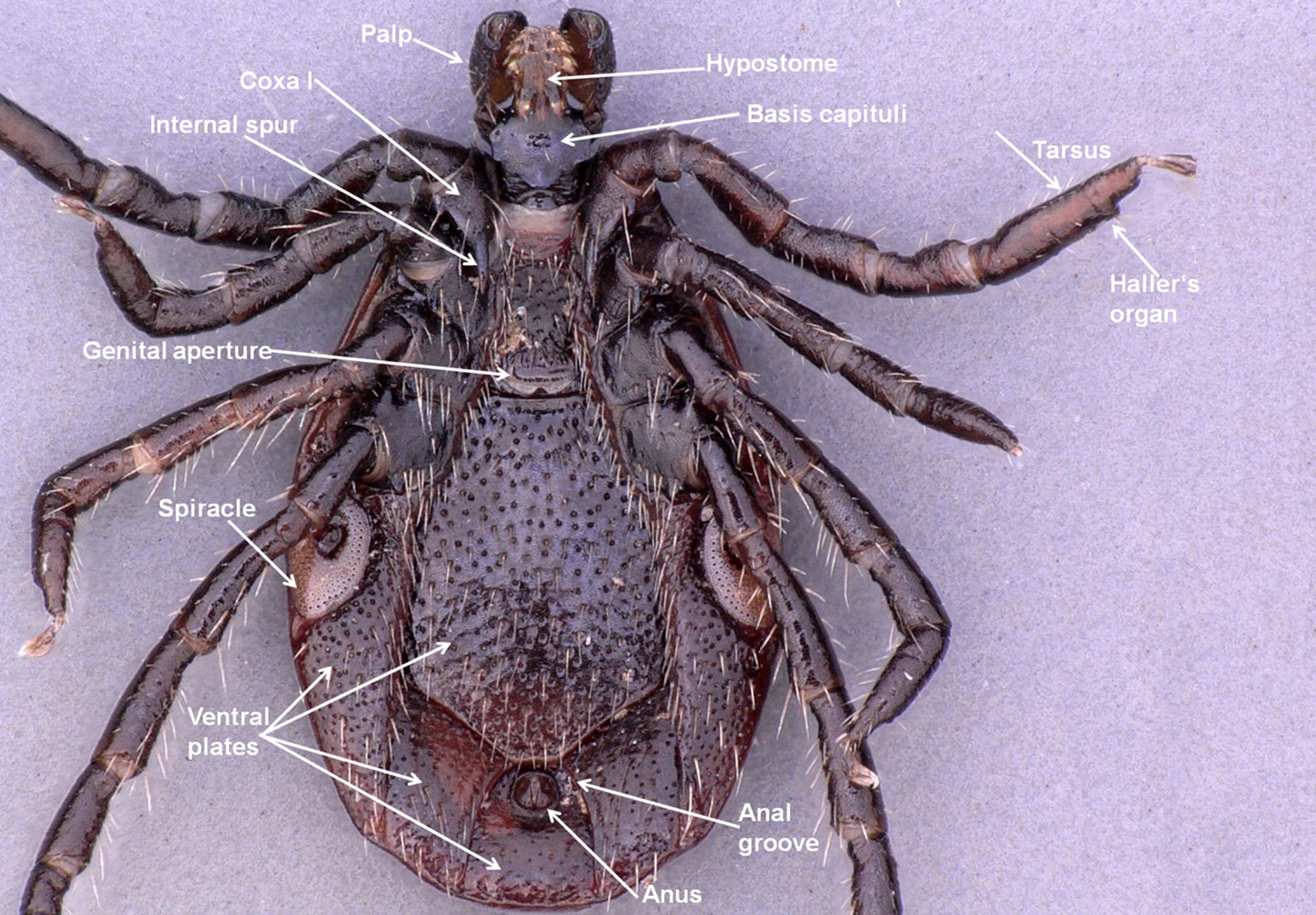
The larva, nymph, and adult (female or male – Figures 2a, 2b, 2c, and 2d) are active stages that require a host (this is not the case for males of the genus Ixodes, which can mate off-host without feeding).17 Larvae are easily recognizable by the presence of only 3 pairs of legs, and absent spiracular and genital apertures (Figures 3a and 3b). Nymphs have 4 pairs of legs and spiracles (Figures 4a and 4b). Adult females have 4 pairs of legs, and spiracles, a genital aperture, and porose areas on the dorsal surface of the basis capituli (Figures 2a and 2b). Adult males have 4 pairs of legs, the scutum covers the entire dorsal surface, and 7 hard sclerotized plates cover the ventral body surface of some species (Figures 2c and 2d).
Types of ticks
All the species known to transmit TBEV have a 3-host life cycle (Figure 1). Each postembryonic life stage requires a blood meal from a suitable host, after which the tick detaches and molts in the leaf litter. The arrows with broken lines in the figure show the potential transmission paths to humans. The line from larvae to humans indicates that transovarial trans-mission from an infected female can happen which results in infective larvae. Infection of the tick can occur when larvae, nymphs, or females feed on an infective host (see below).
The larva, nymph, and adult (female or male – Figures 2a, 2b, 2c, and 2d) are active stages that require a host (this is not the case for males of the genus Ixodes, which can mate off-host without feeding).17 Larvae are easily recognizable by the presence of only 3 pairs of legs, and absent spiracular and genital apertures (Figures 3a and 3b). Nymphs have 4 pairs of legs and spiracles (Figures 4a and 4b). Adult females have 4 pairs of legs, and spiracles, a genital aperture, and porose areas on the dorsal surface of the basis capituli (Figures 2a and 2b). Adult males have 4 pairs of legs, the scutum covers the entire dorsal surface, and 7 hard sclerotized plates cover the ventral body surface of some species (Figures 2c and 2d).
Table 1: Tick species, habitats, and involved hosts in relation to the TBEV subtype and distribution
| Tick species | Main habitat6,17,148 | Virus type | Vector role | References** | |
|---|---|---|---|---|---|
| Host6,17,148 | |||||
| Ixodes (Ixodes) ricinus70,78,91, 138-145 | Exophilic, deciduous and | Polyxenic, | ES, SS | Principal vector in Europe | Radda 1973; Kožuch et al. |
| mixed forests | reptiles, birds, | 1967; Alekseev et al. 1996; | |||
| mammals, | |||||
| human | Demina et al. 2010; Süss | ||||
| 2011; | |||||
| Wojcik-Fatla et al. 2011; | |||||
| Stefanoff et al. 2013; | |||||
| Katargina et al. 2013; Biernat | |||||
| et al. 2014; Drelich et al. 2014; | |||||
| Cuber et al. 2015 | |||||
| Ixodes | Nidicolous, nests and burrows | Birds | ES | Persistence and transmission to white mice; | Lichard and Kožuch 1967; Gresikova and Kaluzova 1997 |
| (Pholeoixodes) arboricola49,50 | considered to be a secondary amplifying vector of TBE | ||||
| virus in wild populations | |||||
| Ixodes | Nests | Birds | SS | Demina et al. 2010 | |
| (Pholeoixodes) | |||||
| lividus140 | |||||
| Ixodes | Nidicolous, nests, | Hedgehogs, wild | ES | Transstadial and transovarial | Radda 1973; Krivanec et al. |
| (Pholeoixodes) | burrows, caves, rock | carnivores, | transmission; TBE virus | 1988; Valarcher et al. 2015; Streissle 1960 | |
| hexagonus62,91,146,147 | shelters, dog | dogs, rarely human | isolates | ||
| kennels and also | Isolated from female and | ||||
| buildings | nymph infesting a hedgehog; | ||||
| a pool of 3 females from red | |||||
| fox | |||||
| Ixodes | Nidicolous, nests, burrows | Hedgehogs, wild carnivores, | ? | Little is known about the vector competence | Radda et al. 1968; Radda 1973 |
| (Pholeoixodes) | dogs | ||||
| canisuga90,91 | |||||
| Ixodes (Scaphixodes) | Nests | Birds | ES | Detection of TBEV; vector competence and importance in transmission cycle unknown | Labuda and Nuttall 2004; Obsomer et al. |
| frontalis52,60,61 | 2013 | ||||
| Ixodes | Endophilic, shady | Small | ES | Nowak-Chmura and Siuda | |
| (Exopalpiger) | mixed and | mammals (ca | Vector and reservoir of TBE virus among the small | 2012; Valarcher et al. 2015 | |
| trianguliceps146,148 | deciduous forests | 50 species), | |||
| birds, and a | mammals | ||||
| viviparous | |||||
| lizard | |||||
| Ixodes (Ixodes) persulcatus139-141 | Exophilic, deciduous and | Polyxenic, | ES, SS, FES | Siberian and Far East subtypes | Demina et al. 2010; Alekseev et al. 1996; Süss 2011 |
| reptiles, birds, | |||||
| mixed forests | mammals, | Principal vector for the Siberian | |||
| human | and Far Eastern subtypes from | ||||
| north-eastern | |||||
| Europe to Russian Far East, | |||||
| China and Japan |
ES, European subtype (TBEV-EU); FES, Far Eastern subtype (TBEV-FE); SS, Siberian subtype (TBEV-Sib)
* Reference for tick habitat and host: Nowak-Chmura and Siuda, 2012; Petney et al., 2012; Guglielmone et al., 2014
** References for tick species involved in TBE virus transmission
Types of hard ticks
Ixodid ticks fall into 2 behavioral groups. Exophilic or non-nidicolous ixodid ticks occur in the open environment and are associated with forests, savannahs, second-growth areas of scrub and brush, grassy meadows, semi-desert, or desert areas. These species are usually not very host specific. Nidicolous or endophilic ixodid ticks live in or near the nests of their hosts, are adapted to highly specialized environments (crevices or other shelters used by their hosts), and tend to be more host-specific.8,15 Many Ixodes species are nidicolous.15 The main vectors of TBEV, I. ricinus and I. persulcatus are exophilic and exceptional both in terms of their large variety of hosts they use as well as the habitats they occupy.18
Click the image above to enlarge
©Nina Littwin
Click the image above to enlarge
details of dorsal morphological features
Click the image above to enlarge
details of ventral morphological features
Click the image above to enlarge
details of dorsal morphological features
Click the image above to enlarge
details of ventral morphological features
Host-finding behavior
Ixodid ticks’ host-seeking behavior is under the control of different abiotic factors that differ according to the region. In temperate and sub-polar regions, seasonal activity is mainly regulated by ambient temperature, changing photoperiod, and incident solar energy, and in the more temperate regions, tick activity is often controlled by saturation deficit and relative humidity, with long-term dry conditions being adverse for survival.15 Those species involved in the transmission of TBEV tend to quest passively or ambush their hosts by climbing onto weeds, grasses, or other lower vegetation to wait for a host nearby passing.
Ixodes ricinus adults can climb as high as 1.5 m on brushy vegetation.19 The immature stages are found lower, up to 70 cm for larvae (O. Kahl, personal communication) and less than 1 m for nymphs.19 Ticks are able to sense a host with their Haller’s organ, which is located on the tarsi I. Haller’s organ possesses chemo-, mechano-, and thermoreceptors that also ensures (together with the receptors on the palps) selection of a suitable feeding site on the host body. The most important stimuli are carbon dioxide (CO2), vibration produced by moving potential hosts, and host temperature. For some species, visual images, host smell, and even noise can stimulate the tick.15,20-22
Feeding behavior
Feeding behavior, even on preferred hosts, is not a uniform process. An ixodid tick may crawl on the host for several hours in search of a suitable feeding site. After attachment, many ixodid ticks secrete cement during the first 1–2 days to secure themselves at the wound site.22
The feeding tick begins salivating into the developing hematoma and sucking blood; phases of salivation and blood sucking alternate.8 Saliva not only plays an important role in the feeding tick’s osmoregulation23 but has also a variety of pharmacological effects. There is an extensive array of antihemostatic, anti-inflammatory, and immunomodulatory proteins and lipids in the tick saliva that suppress the host’s ability to reject the feeding tick.8,23–26 Anticoagulant effects, inhibiting factor Xa, were first shown in I. ricinus in 1898-1899.22,23 In addition, many tick species produce proteins that inhibit thrombin directly or inhibit the conversion of prothrombin to thrombin by inhibiting factor V. Other proteins prevent platelet aggregation or bind, antagonize or degrade important host mediators of pain, itching and inflammation, particularly the host’s own histamine, serotonin, and bradykinin.8,25
Ixodid ticks feed gradually because they must first produce new cuticle to accommodate the massive blood meal.17 Typical attachment periods range from as few as 2 days for larvae to as long as 13 days for females.3,15
An I. ricinus female can reach approximately 450 mg at the end of feeding from approximately 2 mg at the beginning of feeding.21
Drop-off
The controlled timing of drop-off from the host offers important ecological advantages. For non-nidicolous ticks, such drop-off rhythms are synchronized with host behavioral patterns. This tends to disperse fed ticks in optimal habitats where they can develop and reproduce. Photoperiod appears to be the dominant abiotic exogenous factor affecting drop-off patterns. The daily light: dark cycle induces a regular rhythm of feeding and dropping off. Detachment may occur while hosts are inactive in their nests or burrows or, alternatively, it may be coordinated with the period of high host activity.15
Host specificity
Tick species can be either opportunistic or specific with respect to the hosts they choose; both I. ricinus and I. persulcatus are opportunistic species, especially the immatures. For I. ricinus, more than 300 species of vertebrate hosts have been recorded.15,27 Larvae and nymphs of I. ricinus feed readily on lizards, birds, and small mammals, as well as on larger hosts including deer. Adults feed on medium-sized and large mammals, especially ungulates, as well as humans, as do the immature ticks.15 I. persulcatus is more restricted to mammal hosts.28
Questing height is also important. Ticks questing on or near the ground are exposed mostly to small animals, while those questing higher in the vegetation are more likely to encounter larger animals. The extent to which different hosts are utilized depends on host behavior and opportunities for contact, such as foraging range, time of day and time spent foraging, habitats visited, and other factors.15
Acceptance of a vertebrate animal is also dependent on physiological factors and the ability of the ticks to recognize it as a host. Host utilization may be influenced by the ability of ticks to evade or suppress host homeostatic systems and avoid rejection.24
Hard tick ecology, environmental factors
Ticks occur in many terrestrial habitats ranging from cool, arboreal northern forests to hot, arid deserts. Each species, however, has become adapted to the specific types of habitat where it is generally found in highest abundance. All I. ricinus postembryonic stages are exophilic and depend entirely on a suitable combination of climatic variables, making them vulnerable to climate changes and especially to desiccation. Thus, they are mainly found in cool, moist forests.8,21,29,30
Water balance is a critical determinant of a tick’s ability to wait for hosts. Ticks may quest for weeks or even months while waiting for a host. When they have a body water deficit, they retreat to more sheltered, humid micro-environments, such as the rotting vegetation in a meadow or damp leaf litter on the forest floor. They secrete a hygroscopic salivary secretion onto their external mouthparts that collects atmospheric water at relative humidities =80-85% (active water vapor sorption).31 Rehydrated ticks are able to resume host-seeking. Some ticks are able to remain in the questing position for many days without rehydration, while others must return to their humid microenvironments.32 Dense ecotonal vegetation provides shade, increased moisture, protection from intense solar radiation, and plants that support the tick hosts.
There have been various studies showing the relationship between I. ricinus and vegetation type in central Europe33,34 and the capacity of this species to adapt to a large variety of biotopes with low temperature (e.g. Sweden) and high altitudes, up to 1500 m.35-37
Normally, temperature and relative humidity in a burrow, cave, or similar type of shelter are more uniform throughout the year than in the external macroenvironment. The higher relative humidity in such microenvironments is due in part to the presence of hosts, their wastes, and the plant materials they use to construct or line their nests.38 Nidicolous ticks exhibit behavioral patterns that restrict their distribution to these sheltered locations. They avoid bright sunlight and low humidity, the type of conditions prevailing at the entrances of burrows or caves. Confined within these hidden, restricted locations, nidicolous ticks become active when hosts are present. However, when the hosts are absent, they may wait for up to several years for hosts to return, or until they die of starvation.
Diapause
An important physiological trait that enables ticks to survive adverse environmental conditions and conserve energy until conditions improve is diapause as a form of dormancy.39 Diapause is induced by an external cue before adverse conditions occur. It is not terminated by favorable external conditions – as it is the case with quiescence – but there is some diapause development before its termination. During diapause ticks become inactive, reduce their metabolic rates, and do not feed on hosts even when given the opportunity.8,21 Diapause can occur in each life stage, whether it is unfed or engorged. This varies, however, between species and can also differ within a tick species in different geographic areas. As an example, oviposition can be delayed in D. marginatus. Engorged females that feed in late summer, early fall or in winter oviposit only in the following spring.8
Table 2: Animal hosts from which TBEV* has been recovered
| Order/Family | Species | Virus type | |
|---|---|---|---|
| Mammalia: Rodentia | |||
| Muridae | Apodemus agrariusM85,93,150 | FES | |
| Apodemus flavicollis93,138 | ES | ||
| Apodemus sylvaticus93,138 | ES | ||
| Apodemus speciosus151 | FES | ||
| Apodemus argenteus151 | FES | ||
| Myodes rufocanus151 | FES | ||
| Rattus norvegicus151 | FES | ||
| Cricetidae | Microtus agrestis93 | ES | |
| Microtus arvalis93,138 | ES | ||
| Myodes glareolus93,138,150 | ES | ||
| Myodes rufocanus85 | |||
| Myodes rutilus85 | |||
| Sciuridae | Sciurus vulgaris59,138 | ES | |
| Dipodidae | Sicista betulina | ||
| Eulipotyphla | |||
| Erinaceidae | Erinaceus concolor59 | ||
| Erinaceus roumanicus138 | ES | ||
| Talpidae | Talpa europaea59 | ||
| Soricidae | Sorex araneus85,138 | ES | |
| Goats | Capra sp.157-159 | ||
| Sheep | Ovis aries158 | ||
| Bovidaes | Bos taurus158 | ||
| Bison | Bison bonasus72 | FES | |
| Carnivora | |||
| Canidae | Vulpes vulpes90,91,152,153 | ||
| Canis familiaris160 | FES | ||
| Mustelidae | Mustela putorius115 | ES | |
| Artiodactyla | |||
| Cervidae | Cervus elaphus134,154 | ||
| Capreolus capreolus134,155,156 | |||
| Alces alces134 | |||
| Aves (families)** | Virus isolation59,82,161,162: Passeriformes: Acrocephalidae, Bombycillidae, Corvidae, Emberizidae, | ||
| Frigillidae, Hirundinidae, Laniidae, Motacillidae, Muscicapidae, Paridae, Passeridae, Psylloscopidae, Sittidae, Sturnidae, Sylviidae, Turdidae. | |||
| Others: Anatidae, Phasianidae, Picidae, Rallidae, Scolopacidae. | |||
| Transovarial transmission59: Accipitridae, Charadriidae, Columbidae, Emberizidae, Laniidae, Troglodytidae, Turdidae |
ES, European subtype (TBEV-EU); FES, Far-Eastern subtype (TBEV-FE); SS, Siberian subtype (TBEV-Sib)
*Selected references; **Less information available
Seasonal activity
Ixodes persulcatus inhabits mainly coniferous forests of Asia and Eastern Europe, while I. ricinus inhabits deciduous and mixed forests in the British Isles, in Continental Europe, and western Asia.8,28,40–42 Ixodes persulcatus adult females and eggs are unable to survive the winter, however, that I. persulcatus larvae and nymphs, whether unfed or engorged, are able to overwinter. In contrast, eggs as well as unfed and satiated females of I. ricinus are capable of overwintering, a principal difference between the life cycles of the 2 tick species. Vector tick activity is well correlated with the seasonal pattern of TBE occurrence. In such a focus, it is common for 2–3% of the ticks to be virus-infected.43 In Northern and Central Europe, the seasonal activity of I. ricinus often has 2 peaks, one in spring (May–June) and the other one at the end of summer (September-October).
Unfed Dermacentor reticulatus adults are mostly active in spring and autumn, occasionally in winter but usually not in summer (June to early August).44-46
Tick species involved with TBEV transmission
Of the 54 species of ixodid ticks known from the Western Palearctic,47 8 species from 3 genera are known to be able to transmit TBEV, and the virus has been isolated from at least 14 other species (Table 1). Ixodes ricinus, the most commonly encountered European tick species, is considered to be the principal vector of TBEV there.48 Lichard and Kozuch49 were able to show TBEV persistence and transmission to white mice by Ixodes arboricola, which is considered a secondary amplifying vector of TBEV.50 Ixodes persulcatus is also known to transmit TBEV.51,52 It is the adult female I. persulcatus, which infects humans with TBEV and other zoonotic pathogens. Neither the larval nor the nymphal stage often attach to humans. 7 Both D. marginatus and D. reticulatus are also vectors of TBEV.53-55
Haemaphysalis concinna is a known vector of TBEV as well.56,57 Evidence for the vectorial capacity of Haemaphysalis inermis for TBEV is available from Nosek et al.58
The virus has been isolated in the Czech Republic from female and nymphal I. hexagonus infesting a hedgehog.62 TBEV also has been detected in Haemaphysalis punctata.63,64
The role of Dermacentor ticks (Table 1) in the circulation of TBEV in the environment is unclear and poorly studied.65,66 D. reticulatus appears to be spreading and population density increasing during recent decades.65-69 In eastern Poland, the mean prevalence of infection with TBEV found in questing adult D. reticulatus was 10.8% (range 7.3–14.3% in infected areas): This is considerably higher than the prevalence found in questing adult I. ricinus (1.6%, range 0.7–4.3% in infected areas).70
Prevalence of TBEV in questing adult D. reticulatus ticks from Białowieża Primeval Forest was similar (1.58%)71 to that in questing I. ricinus (1.30%),72 as was the case in Moldova (adult I. ricinus 3.8%, adult D. reticulatus 3.9%, but adult Haemaphysalis punctata 8.8%).73 The natural occurrence of TBEV in a D. reticulatus tick population has also been proven for Germany during 2016 to 2018 by isolation of several TBEV strains from this tick species in a natural focus.74
The differences in TBEV prevalence in the various vector species remain puzzling. Questing I. ricinus usually have a very low prevalence of the virus, ranging from no virus in many areas to less than 1% in most others, and rarely reaching 2–5%, in unfed adults.75-79 Knap and Avsic-Zupanc80 showed that over a 4-year period, the prevalence was at the expected low level in the 8 areas studied, but that no area was consistently positive for the virus. This may be related to the frequently low sample sizes (14/30 samples had fewer than 300 specimens).
Prevalence of the virus in feeding ticks, although very variable, can be substantially higher.79 Waldenström et al.81 showed a low prevalence (0.5%) in nymphs and larvae feeding on migratory birds in Sweden, while Kazarina et al.82 detected 14% nymphs and 7% larvae of I. ricinus on migratory birds infected in Latvia. Data for I. persulcatus are more variable. Korenberg and Kovalevskii83 reported a high TBEV prevalence in unfed adults, ranging from 10.9% to 38.7% over 6 years (mean 26.2%) in unfed adults in the Pre-Ural Region, whereas the prevalence in the Primorskii Region of the Russian Far-East ranged from a little over 1% to over 9% from 1970 to 1990, and in the Khabarovsk Region from 3.4% to 9.4% over 4 years.84 In the Novosibirsk Region, the prevalence of TBEV in unfed adult I. persulcatus was 3.6%, with 0.8% being pathogenic to laboratory mice.85 In the same study, 3.3% of questing adult I. pavlovskyi were infected with the virus with 1.8% of the isolates being pathogenic. Information on less commonly encountered vectors is rarely available and sample sizes are usually low, making such data unreliable (e.g., Kim et al.)86 Long-term studies and statistical analyses showed that higher average temperatures during the summer-autumn period may lead to higher levels of TBEV found in ticks and consequently increase the risk for humans to develop symptomatic TBE following an infected tick bite.87
Vertebrate hosts
The prevalence of antibodies to TBEV in hosts is quite variable.81 TBEV has been found in numerous mammal species from different families, as well as in a large number of passerine and non-passerine bird species (Table 2). Virus infection was demonstrated by antibodies to the virus or viral ribonucleic acid (RNA) detection in a wide variety of bird species,81,82,88,89 with virus isolation from Turdus pilaris (fieldfare) and Acrocephalus dumetorum (Blyth’s reed warbler) opening the possibility of virus transfer to new foci during bird dispersal or migration.88 Viremia has been induced experimentally in birds, reaching levels sufficient to infect feeding ticks.59 Generally speaking, findings of TBEV in animals, whether indirect or direct, do not mean that much eco-epidemiologically. Only the demonstration of reservoir competence indicates an active role in the perpetuation of TBEV.
Red foxes (Vulpes vulpes) are known to be reservoir competent for TBEV.90,91 Although I. hexagonus is a proven vector of TBEV, little is known about the vector competence of the fox tick I. canisuga.
In recent years, the detection of viral RNA in hosts has become possible. Tonteri et al.105, in Finland, detected the European (TBEV-EU) and Siberian (TBEV-Sib) subtypes in M. glareolus, TBEV-Sib in the shrew Sorex araneus, and TBEV-EU in Microtus agrestis. Achazi et al.93detected TBEV RNA in rodent brain tissue in prevalence up to 20% in TBE non-risk as well as in risk areas in east-German Federal States. In the Novosibirsk region of Siberia, where I. persulcatus and I. pavlovskyi are the main TBEV vectors, the prevalence of TBEV viral RNA in 5 small mammal species was extremely high.85 It ranged from 35.3% for A. agrarius organs to 82.2% for Myodes rutilus blood, with a mean value for all species and tissues of 62.1%. All 3 virus subtypes were represented. In addition to small mammal hosts, larger wild and domestic animals frequently have high antibody prevalence. Because they feed large numbers of vector ticks, they can be used as sentinels for the occurrence of TBEV in a given area.
TBEV transmission
Nuttall et al.91 noted: “Reciprocal interactions of parasites transmitted by blood-sucking arthropod vectors have been studied primarily at the parasite-host and parasite-vector interface. The third component of this parasite triangle, the vector-host interface, has been largely ignored.”
The adult female tick is considered to play only a minor role in virus circulation. Tick males, which either do not feed or feed for only a short time, might also be involved in virus transmission.96 TBEV invades all tick tissues, including the salivary glands and ovaries,95 thus it may be transmitted by ticks in the following ways: 1) via saliva, 2) transovarially (vertically), and 3) sexually.40,97–99
TBEV transmission from vector ticks to hosts via saliva
Certain species of ticks are vectors and reservoirs of TBEV, and they can transmit the virus already when they start feeding43,100 with viral particles contained in the saliva, which the ticks release into the host tissues.40
TBEV is present in the alveolar cells of the salivary glands of D. marginatus and H. inermis females in as few as 5 days after their feeding on viremic white mice.55Also certain vertebrates, so-called reservoir hosts, are important for the amplification of the virus and are together with vector ticks the basis for the heteroxenous TBEV perpetuation.101
Viremic transmission from hosts to feeding ticks
Ticks become infected with TBEV while they feed on a viremic host.98,99,102Nosek et al.103,104 proved that a viremia in a host lower than 101 mouse LD50./0.03 ml was insufficient to cause infection in ticks. In individual engorged I. ricinus ticks, the virus titer was 101-104 mouse LD50/0.03 ml. Viremic white mice served as virus donors.103,104 Grešíková and Nosek105 demonstrated the persistence of TBEV in H. inermis (from larva to nymph) and then the transmission from H. inermis nymphs to white mice. Viremia surpassing the threshold values of infectivity for tick vectors was also found in some juvenile and adult Myodes rufocanus, M. rutilus, and Micromys minutus. The viremia level depends on the rodent species and age and exhibits individual variability.106
Co-feeding transmission
TBEV transmission is also possible from infected to non-infected ticks during feeding close to each other on a non-viremic host.98,102 Cellular infiltration of tick feeding sites, and the migration of cells from such sites, can provide a vehicle for transmission between co-feeding ticks that is independent of host viremia.102 The non-viremic route of transmission between co-feeding ticks can even occur in rodents that are already immune to TBEV.108 The degree of co-feeding virus transmission may be influenced by local climatic factors that affect the seasonal timing of tick host-seeking activity and, as such, can be used to predict the focal distribution of TBEV.107,109
Transovarial transmission
Another possible way for ticks to transmit TBEV involves transovarial transmission and transstadial persistence (see below), which were described for the first time as early as 1940.110 However, only some eggs in the batch of a TBEV-infected vector tick female become infected.111 In addition, virus can partly be lost during transition from stage to stage,112 and not all tick individuals reach the next life stage irrespective of the presence or absence of the pathogen. Danielova and Holubova113 found that only 0.23% of larvae coming from infected females were TBEV-positive. Other studies showed that 0.58% to 0.75% of the larvae were transovarially infected. Thus, the rate of transovarial transmission remains below 1%. Nuttall et al.114 suggest that transovarial transmission is important for the maintenance of a natural focus even if it occurs at a very low rate.
Danielova et al.76 detected TBEV in 2 out of 647 flagged larvae of I. ricinus, which indicates transovarial transmission.
Transstadial persistence
TBEV was not detectable in I. ricinus nymphs 14 days after molting from larvae that had engorged on viremic A. flavicollis, but TBEV was present in these ticks 2 months post ecdysis. Many nymphs contained the virus, indicating that the latter undergoes an eclipse phase during metamorphosis.
Sexual transmission in ticks
Transmission of TBEV from males to females116 is successful in only 10% of copulations in infected I. persulcatus, but it may provide notable support for the transfer of the virus to the following generation of ticks if transovarial transmission follows. A mathematical model of sexual transmission of the virus117 was developed long before determining that such a sort of transmission occurs. Virus exchange between a non-engorged female and an infected male of I. persulcatus that ‘feeds’ on (i.e., attaches to) the female before or after copulation is quite probable, and it has been proven that the saliva of starved males contains a fairly large amount of virus, sufficient for infecting not only animals118 but also humans. The feeding of I. persulcatus males on females with which they later copulate can be observed in 2–10% of cases.118
Vertical TBEV transmission in vertebrates
TBEV transmission from mother to her offspring in small rodents, e.g., red voles (M. rutilus), was shown for naturally infected reservoir hosts as well as after experimental infection with different sublethal doses of the virus.119 TBEV RNA was detected in up to 90% of the newborn rodents, 240–280 days after experimental infection of their parents, by real-time polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and bioassays. The small amounts of TBEV RNA detected in the embryos, placenta, and blood serve as evidence of prenatal transmission. Postnatal transfer of the virus might occur through the rodent’s milk. Vertical virus transmission may occur before, during, and/or after birth of the baby rodents with a high frequency. In natural foci, this could ensure long-term persistence of TBEV in mammal hosts without involving any arthropod vectors.119
Non-reservoir hosts do not directly participate in virus transmission but can play an important role in the maintenance of natural foci. The density of reservoir-incompetent hosts may have either a positive effect on virus transmission, by amplifying the tick population, or a negative (‘dilution’) effect, as tick bites on a non-reservoir host cannot lead to virus transmission.98,120
Alimentary route of transmission
Humans mostly become infected with TBEV via tick bites, but viral transmission is also possible via the consumption of unpasteurized goat, cow and sheep milk.43 Approximately 1% of all TBEV infections in humans are probably acquired by consuming infected unpasteurized milk and milk products from infected livestock, particularly goats.121
Outbreaks due to alimentary virus transmission are known from Eastern, Central and Southern Europe,122,123 and must be considered particularly in cases of local epidemics.123–125
The natural cycle
The natural cycle of TBEV is highly complex, and many details remain obscure. The three prevailing TBEV subtypes overlap in some areas, they all have multiple mammalian reservoir hosts and various tick vectors, and in some areas these subtypes occur sympatrically. Humans are not included in these natural cycles but may enter those transmission cycles inadvertently.
Small mammals as a reservoir and vector ticks play a central role in the natural cycle of TBEV, but non-reservoir hosts such as birds and large vertebrates, such as wild ungulate species, or foxes, may also indirectly contribute to the spread and maintenance of TBEV. Additionally, changing climatic patterns, as well as changes in ecosystems, may not only affect the spatial distribution of TBEV, but also the maintenance of small natural TBEV foci.128,129 Small rodents such as A. flavicollis are important hosts for the larvae of I. ricinus, the probably most important TBEV amplifying host in Central Europe. Apodemus flavicollis temporarily develops high virus titers necessary to infect ticks. Detailed studies by Radda et al.,90,115 who trapped small rodents and collected the engorged ticks in a natural TBE focus for 2 years, showed that given certain prerequisites are fulfilled (high numbers of rodents, vector tick larvae and nymphs feeding on these rodents), such a natural TBEV focus is able to sustain itself without any significant input of other hosts. This may explain why many of these natural foci are stable, but restricted to small areas, and why they harbor TBEV-positive ticks over a long period of time. Forest structure, especially deforestation and reforestation, are known to have a huge impact on ticks and vertebrate reservoir hosts for many tick-borne pathogens.130,131
Experimental transstadial maintenance of TBEV in D. marginatus and D. reticulatus ticks emphasizes the role of both species. TBEV infection and transmission rates in Dermacentor species to hosts are somewhat lower than in species of the genera Ixodes and Haemaphysalis.54 Feeding larvae and nymphs of I. persulcatus may become infected with TBEV if the virus titer in the host blood reaches at least 3.0 log10 LD50/0.03 mL.132 Such levels of viremia occur only in small rodents and are a critical factor in the virus circulation between vertebrates and ticks in natural foci. In small rodents, the infection is asymptomatic.91
TBEV has been isolated from a wide range of birds from many different families, including migratory species, which may be important for the distribution of the virus. A common strategy for migratory birds is to rest at certain stopover sites along their routes. At these sites, the birds can be infested with ticks or engorged ticks can detach after engorgement. Sándor et al.133 detected 4 different tick species on 11 different bird species in the Danube Delta, including larvae, nymphs, and females of I. ricinus.
A high variability is found between areas and years with respect to viral prevalence in both vertebrate hosts and vector tick populations, while consistent differences between vectors. For example, the generally higher TBEV prevalence in I. persulcatus compared with those in I. ricinus may relate to the ecology/biology of the individual vectors. The complexity is well defined by the various mathematical models aimed at exploring the dynamics of TBEV ecology.98,136,137 Hartemink et al.137 list 19 parameters based on field data to define the basic reproduction number (Ro) of tick-borne infections, while Rosà et al.98 list 32 parameters in a more comprehensive model. Unfortunately, no single study has been able to comprehensively measure all the parameters needed to test these models, although approximations are available.
Contact:
lydiachitimia@gmail.com
Citation:
Chitimia-Dobler L, Mackenstedt U, Kahl O. Transmission/Natural cycle. Chapter 3. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi: 10.33442/26613980_3-6
References
- de la Fuente J, Estrada-Pena A, Venzal J, Kocan K, Sonenshine D. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008;13:6938-46.
- Estrada-Peña A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685-715.
- Balashov Y. Bloodsucking ticks (Ixodoidea) – vectors of diseases of man and animals. Misc Publ Entomol Soc. 1972;8:163-376.
- Skuballa J, Petney T, Pfäffle M, Taraschewski H. Molecular detection of Anaplasma phagocytophilum in the European hedgehog (Erinaceus europaeus) and its ticks. Vector Borne Zoonotic Dis. 2010;10:1055-7.
- Pfäffle M, Littwin N, Muders S, Petney T. The ecology of tick-borne diseases. Int J Parasitol. 2013;43:1059-77.
- Beati L, Klompen H. Phylogeography of Ticks (Acari: Ixodida). Annu Rev Entomol. 2019;64:379-397.
- Guglielmone A, Robbins R, Apanaskevich D, Petney T, Estrada-Peña A, Horak I. The hard ticks of the world (Acari: Ixodida: Ixodidae). Heidelberg: Spinger; 2014.
- Nicholson W, Sonenshine D, Lane R, Uilenberg G. Ticks (Ixodida). In: Medical and Veterinary Entomology. 2nd Ed. Eds. Mullen GR, Durden LA. 2009.
- Barker S.C., Burger T.D. Two new genera of hard ticks, Robertsicus n. gen. and Archaeocroton n. gen., and the solution to the mystery of Hoogstraal’s and Kaufman’s “primitive” tick from the Carpathian Mountains. Zootaxa. 2018;4500 4:543-552.
- Petney T, Robbins R, Guglielmone A, et al. A look at the world of ticks. Parasitology Research Monographs. 2011;2:283-96.
- Sonenshine D, Roe RM. (eds) Biology of Ticks, 2nd ed., Volume 1 and 2, Oxford: Oxford University Press; 2013.
- Guglielmone A, Nava S. Names for Ixodidae (Acari: Ixodoidea): valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names–with notes on confusions and misidentifications. Zootaxa. 2014;24:1-256.
- Clifford C.M., Sonenshine D.E., Keirans J.E., Kohls G.M. Systematics of the subfamily Ixodinae (Acarina: Ixodidae) 1. The subgenera of Ixodes. Entomol. Soc. Ann. 1973;66:489–500.
- Filippova N.A. Ixodid Ticks of the Subfamily Amblyomminae. Izd Nauka. Leningrad, Russia. 1977.
- Sonenshine D, Lane R, Nicholson W. Ticks (Ixodida). Med Vet Entomol. 2002;10:517-58.
- Sands A.F., Apanaskevich D.A., Matthee S., Horak I.G., Harrison A., Karim S., Mohammad M.K., Mumcuoglu K.Y., Rajakaruna R.S., Santos-Silva M.M., Matthee C.A. Effects of tectonics and large-scale climatic changes on the evolutionary history of Hyalomma ticks. Mol Phylogenet Evol. 2017;114:153-165.
- Petney T, Pfäffle M, Skuballa J. An annotated checklist of the ticks (Acari:Ixodida) of Germany. Syst Appl Acarol. 2012;17:115-70.
- Moshkin M, Novikov E, Tkachev S, Vlasov V. Epidemiology of a tick-borne viral infection: theoretical insights and practical implications for public health. BioEssays. 2009;31:620–8.
- Liebisch A, Liebisch G. Biologie und Ökologie der Zecken. In: Einheimische Zeckenborreliose (Lyme-Krankheit) bei Mensch und Tier. 4th Ed. Eds Horst H, Liebisch A. Balingen: Spitta; 2003.
- Waladde S, Rice M. The sensory basis of tick feeding behavior. In: Physiology of ticks. Eds Obenchain F, Galun R. Oxford: Pergamon Press; 1982.
- Guetard M. Ixodes ricinus: morphologie, biologie élevage, données bibliographique. Toulouse: Thése dr. vet. ENV; 2001.
- Mehlhorn H. Encyclopedic reference of parasitology. Berlin, Heidelberg: Springer; 2001.
- Mao H, Kaufman WR. DNA binding properties of the ecdysteroid receptor in the salivary gland of the female ixodid tick, Amblyomma hebraeum. Insect Biochem Mol Biol. 1998;28:947-57.
- Ribeiro J. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463-78.
- Ribeiro J. Ribeiro JMC. Role of saliva in tick/host interactions. Exp Appl Acarol. 1989;7:15-20.
- Turni C, Lee R, Jackson L. Effect of salivary gland extracts from the tick, Boophilus microplus, on leucocytes from Brahman and Hereford cattle. Parasite Immunol. 2002;24(7):355-61.
- Andersson J. Epizootiology of Lyme Borreliosis. Scand J Infect Dis. 1991;77:23-34.
- Balashov Y. Distribution of ixodid ticks (Acarina, Ixodidae) over landscapes within their ranges. Entomol Rev. 1997;77:625-37.
- Estrada-Peña A, Mihalca A, Petney T, (eds). Ticks of the Western Palearctic. Heidelberg: Springer; 2017.
- Gage K, Burkot T, Eisen R, Hayes E. Climate and vector-borne diseases. Am J Prev Med. 2008;35:436-45.
- Gaede K., Knülle W. On the mechanism of water vapour sorption from unsaturated atmospheres by ticks. J Exp Biol. 1997;200:1491–8.
- Knülle W., Rudolph R.D. Humidity relationships and water balance of ticks. In Physiology of Ticks (ed. F. D. Obenchain. and R. L. Galun), pp. 43–70. Oxford: Pergamon Press; 1982.
- Daniel M, Kolar J. Using satellite data to forecast the occurrence of the common tick Ixodes ricinus. J Hyg Epidemiol Microbiol Immunol. 1990;34(3):243-52.
- Daniel M, Kolar J; Zeman P, Pavelka K, Sadlo J. Predictive map of Ixodes ricinus high incidence habitats and a tick-borne encephalitis risk assessment using satellite data. Exp Appl Acarol. 1998;22:417-33.
- Perez C, Rodhain F. Biologie d’Ixodes ricinus, I. Ecologie, cycle evolutif. Bull Soc Pathol Exot. 1977;2:187-92.
- Perez C, Rodhain F. Biologie d’Ixodes ricinus, II. Incidence epidemiologique. Bull Soc Pathol Exot. 1977;2:193-201.
- Tälleklint L, Jaenson T. Tälleklint L, Jaenson TGT. Increasing geographical distribution and density of Ixodes ricinus (Acari: Ixodidae) in central and northern Sweden. J Med Entomol. 1998;4:521-6.
- Burda H, Šumbera R, Begall S. Microclimate in burrows of subterranean rodents – revisited. In: Subterranean rodents: News from underground. Eds Begall S, Burda H, Schleich C. Berlin Heidelberg: Springer Verlag; 2007.
- Gray J, Kahl O, Lane RS, Levin ML, Tsao JI. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick-Borne Dis. 2016 7:992-1003.
- Filippova N. Taiga tick Ixodes persulcatus Schulze (Acarina, Ixodidae) Morphology, Systematics, Ecology, Medical importance. [In Russian]. Leningrad: Nauka; 1985.
- Korenberg E. Seasonal population dynamics of Ixodes ticks and tick-borne encephalitis virus. Exp Appl Acarol. 2000;24:665–81.
- Naumov R. The longevity of the tick Ixodes ricinus (Acari: Ixodidae) in Central Russia. [In Russian]. Parazitologia. 2006;40:384-95.
- Gaidamovich S. Tick-borne Flavivirus infections. Exotic Viral Infect. 1995.
- Chitimia-Dobler L. Spatial distribution of Dermacentor reticulatus in Romania. Vet Parasitol. 2015;214:219-23.
- Karbowiak G. The occurrence of the Dermacentor reticulatus tick – its expansion to new areas and possible causes. Ann Parasitol. 2014;60:37–47.
- Kiewra D, Czulowska A, Lonc E. Winter activity of Dermacentor reticulatus (Fabricius, 1794) in the newly emerging population of Lower Silesia, south-west Poland. Ticks Tick Borne Dis. 2016;6:1124-7.
- Estrada-Peña A, Mihalca A, Petney T, (eds). Ticks of the Western Palearctic. Heidelberg: Springer; 2018.
- Süss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine. 2003;21 Suppl 1:S19-35.
- Lichard M, Kozuch O. Persistence of tick-borne encephalitis virus in nymphs and adults of Ixodes arboricola and its transmission to white mice. Acta Virol. 1967;11:480.
- Grešíková M, Kaluzova M. Biology of tick-borne encephalitis virus. Acta Virol. 1997;41:115–24.
- Nuttall P, Labuda M. Tick-borne encephalitis subgroup. In: Ecological dynamics of tick-borne zoonoses. Eds Sonenshine D, Mather T. 1994:351-91.
- Labuda M, Nuttall P. Tick-borne viruses. Parasitology. 2004;129 (S1):S221-45.
- Hoogstraal H. Ticks in relation to human diseases caused by viruses. Ann Rev Entomol. 1966;11:261–308.
- Kožuch O, Nosek J. Transmission of tick-borne encephalitis (TBE) virus by Dermacentor marginatus and reticulatus ticks. Acta Virol. 1971;15:334.
- Nosek J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in central Europe. Folia Parasitol. 1972;19:93-102.
- Kožuch O, Nosek T. Experimental transmission or tick-borne encephalitis (TBE) virus by Haemaphysalis concinna Acta Virol. 1980;24:377.
- Khazova T, Iastrebov V. Combined focus of tick-borne encephalitis, tick-borne rickettsiosis and tularaemia in the habitat of Haemaphysalis concinna in south central Siberia. [In Russian] Zh Mikrobiol Epidemiol Immunobiol. 2001:78–80.
- Nosek J, Ciampor F, Kožuch O, Rajcáni J. Localization of tick- borne encephalitis virus in alveolar cells of salivary glands of Dermacentor marginatus and Haemaphysalis inermis ticks. Acta Virol. 1972;16:493-7.
- Hubálek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res. 2012;111:9-36.
- Hillyard P. Ticks of north-west Europe. London: Linnaean Society; 1996.
- Obsomer V, Wirtgen M, Linden A, et al. Spatial disaggregation of tick occurrence and ecology at a local scale as a preliminary step for spatial surveillance of tick-borne diseases: general framework and health implications in Belgium. Parasit Vectors. 2013;6:1.
- Krivanec K, Kopecky E, Tomkova E, Grubhoffer L. Isolation of TBE virus from the tick Ixodes hexagonus. Folia Parasitol. 1988;35:273–6.
- Grešíková M. Studies on tick-borne arboviruses isolated in Central Europe. Biological works. Slovak Acad Sci Bratislava. 1972;p.9.
- Grešíková M, Calisher Grešiková M, Calisher CH. The Arboviruses: Epidemiology and ecology. Vol. IV, CRC Press, Inc., Boca Raton, Florida, 1988;p.177.
- Karbowiak G. The occurrence of the Dermacentor reticulatus tick – its expansion to new areas and possible causes. Ann Parasitol. 2014;60:37–47.
- Karbowiak G, Kiewra D. New locations of Dermacentor reticulatus ticks in Western Poland: the first evidence of the merge in reticulatus occurrence areas? Wiad Parazytol. 2010;56:333–40.
- Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. Int J Med Microbiol. 2006; 296:149-56.
- Chitimia-Dobler L. Spatial distribution of Dermacentor reticulatus in Romania. Vet Parasitol. 2015;214:219-23.
- Rubel F, Brugger K, Pfeffer M, et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224-33.
- Wojcik-Fatla A, Cisak E, Zając V, Zwoliński J, Dutkiewicz J. Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick Borne Dis. 2011;2:16-9.
- Biernat B, Karbowiak G, Werszko J, Stańczak J. Prevalence of tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks from natural and urban environment, Poland. Exp Appl Acarol. 2014;64:543-51.
- Biernat B, Karbowiak G, Stańczak J, Masny A, Werszko J. The first detection of the tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks collected from the lowland European bison (Bison bonasus bonasus L.). Acta Parasitol. 2016;61:130-5.
- Ponomareva E, Mikryukova T, Gori A, et al. Detection of Far- Eastern subtype of tick-borne encephalitis viral RNA in ticks collected in the Republic of Moldova. J Vector Borne Dis. 2015;52:334.
- Chitimia-Dobler L, Lemhöfer G, Król N, Bestehorn M, Dobler G, Pfeffer M. Continuous isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit Vectors. 2019;12:90. doi: 10.1186/s13071-019-3346-6
- Nosek J, Kožuch O, Grulich I. The structure of tick-borne encephalitis (TBE) foci in Central Europe. Oecologia. 1970;5:61-73.
- Danielová V, Daniel M, Schwarzová L, et al. Integration of a Tick-Borne Encephalitis Virus and Borrelia burgdorferi sensu lato into Mountain Ecosystems, Following a Shift in the Altitudinal Limit of Distribution of Their Vector, Ixodes ricinus (Krkonoše Mountains, Czech Republic). Vector Borne Zoonotic Dis. 2010;10:223-30.
- Burri C, Bastic V, Maeder G, Patalas E, Gern L. Microclimate and the zoonotic cycle of tick-borne encephalitis virus in Switzerland. J Med Entomol. 2011;48:615-27.
- Drelich A, Andreassen Å, Vainio K, Kruszyński P, Wąsik T. Prevalence of tick-borne encephalitis virus in highly urbanized and low risk area in Southern Poland. Ticks Tick Borne Dis. 2014;5:663-7.
- Imhoff M, Hagedorn P, Schulze Y, Hellenbrand W, Pfeffer M, Niedrig M. Review: Sentinels of tick-borne encephalitis risk. Ticks Tick Borne Dis. 2015;6:592-600.
- Knap N, Avšič-Županc T. Factors affecting the ecology of tick- borne encephalitis in Slovenia. Epidemiol Infect. 357-66.
- Nosek T, Kožuch O, Ernek E, Lichard M. The importance of goats in the maintenance tick-borne encephalitis virus in nature. Acta Virol. 1967;11:470.
- Nosek T, Kožuch O, Ernek E, Lichard M. Ubertragung des Zeckenenzephaliltis Virus durch die Weibchen von Ixodes ricinus und Nymphen Haemaphysalis inermis auf der Rehkitzen (Capreolus capreolus). Zbl Bakt I Orig. 1967;203:162.
- Grešíková M, Nosek J. Isolation of tick-borne encephalitis virus from Haemaphysalis inermis ticks. Acta Virol. 1966;10:359-61.
- Kožuch O, Chunikhin S, Grešíková M, et al. Experimental characteristics of viremia caused by two strains of tick-borne encephalitis virus in small rodents. Acta Virol. 1981;25:219- 24.
- Randolph S, Miklisová D, Lysy J, Rogers D, Labuda M. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177-86.
- Labuda M, Kozuch O, Zuffova E, Eleckova E, Hails R, Nuttall P. Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology. 1997;235:138-43.
- Randolph S, Green R, Peacey M, Rogers D. Seasonal synchrony: the key to tick-borne encephalitis foci identified by satellite data. Parasitology. 2000;121:15-23.
- Pavlovsky E, Soloviev V. Experimental investigation of the tick-borne encephalitis virus circulation in the tick-vector organism (Ixodes persulcatus). [In Russian]. Archiv Biol Nauk. 1940;59:111-7.
- Ilienko V, Gorozhankina T, Smorodintsev A. Main reguliers of transovarial transmission of tick-borne encephalitis virus by tick vectors. [In Russian] Med Parazitol. 1970;3:263-8.
- Benda R. The common tick Ixodes ricinus L. as a reservoir and vector of tick-borne encephalitis. I. Survival of the virus (strain B3) during the development of the tick under laboratory conditions. J Hyg Epidemiol Microbiol Immunol. 1958;2:314-30.
- Danielova V, Holubova J. Transovarial transmission rate of tick-borne encephalitis virus in Ixodes ricinus Mod Acarol. 1991;2:7-10.
- Nuttall P, Jones L, Labuda M, Kaufmann R. Adaptation of arbovirus to ticks. J Med Entomol. 1994;31:1-9.
- Radda A, Hofmann H, Pretzmann G. Threshold of viraemia in Apodemus flavicollis for infection of Ixodes ricinus with tick-borne encephalitis virus. Acta Virol. 1969;13:74-7.
- Chunikhin S, Stefutkina L, Korolev M, Reshetnikov I, Khozinskaya G. Sexual transmission of tick-borne encephalitis virus in ixodids (Ixodidae) [In Russian]. Parazitologia. 1983;17:214-5.2015;143:2059-67.
- Waldenstrom J, Lundkvist A, Falk K, et al. Migrating birds and tick-borne encephalitis virus. Emerg Infect Dis. 2007;13:1215.
- Kazarina A, Japiņa K, Keišs O, et al. Detection of tick-borne encephalitis virus in I.ricinus ticks collected from autumn migratory birds in Latvia. Ticks Tick Borne Dis. 2015;2:178- 80.
- Korenberg E, Kovalevskii Y. Main features of tick-borne encephalitis eco-epidemiology in Russia. Zentralbl Bakteriol. 1999;289:525-39.
- Korenberg E, Horakova M, Kovalevsky J, Hubalek Z, Karavanov A. Probability models of the rate of infection with tick-borne encephalitis virus in Ixodes persulcatus ticks. Folia Parasitol. 1992;39:85-92.
- Bakhvalova V, Chicherina G, Potapova O, et al. Tick-borne encephalitis virus diversity in ixodid ticks and small mammals in south-western Siberia, Russia. Vector Borne Zoonotic Dis. 2016;16:541-9.
- Kim S, Yun S, Han M, et al. Isolation of tick-borne encephalitis viruses from wild rodents, South Korea. Vector Borne Zoonotic Dis. 2008;8:7-14.
- Daniel M., Danielová V., Fialová A., Malý M., Kříž B., Nuttall P.A. Increased relative risk of tick-borne encephalitis in warmer weather. Front Cell Infect Microbiol. 2018;8: doi: 10.3389/fcimb.2018.00090.
- Mikryukova T, Moskvitina N, Kononova Y, et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick Borne Dis. 2014;5:145-51.
- van Tongeren H. Viraemia and antibody response of the mallard (Anas platyrhynchos) to infection with tick-borne encephalitis virus. J Comp Pathol. 1983;4:521-30.
- Radda A, Kunz C, Hofmann H. Nachweis von Antikörpern in Wildseren zur Erfassung von Herden des Virus der Frühsommer-Meningo-Enzephalitis in Niederösterreich. Zentralbl Bakteriol. 1968;208:88-93.
- Radda A. Die Zeckenenzephalitis in Europa. Angewandte Zool. 1973;60:409-61.
- Tonteri E. Tick-borne encephalitis virus in wild rodents in winter, Finland, 2008–2009. Emerg Infect Dis 2011,17.
- Achazi K, Růžek D, Donoso-Mantke O, et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2011;11:641-7.
- Nutall P. Displaced tick-parasite interactions at the host interface. Parasitology. 1998;116:S65-72.
- Karbowiak G, Biernat B. The role of particular tick development stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 2. Tick-borne encephalitis virus. Ann Parasitol. 2016;62:3-9.
- Korenberg E, Ivanova L, Yurkova E. Epidemicity rate of tick-borne encephalitis natural foci (range of limits). Med Parazitol. 1986;2:35-9.
- Labuda M, Randolph S. Survival of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentralbl Bakteriol. 1999;288:51 3-24.
- Rosa R, Pugliese A, Norman R, Hudson P. Thresholds for disease persistence in models for tick-borne infections including nonviraemic transmission, extended feeding and tick aggregation. J Theor Biol. 2003;224:359-76.
- Satz N. Frühsommermeningenzephalitis (FMSE). Huber; 2006.
- Okulova N, Chunikhin S, Vavilova V, Mayorova A. The site of tick’s infecting bite and severity of encephalitis. Med Parazitol. 1989;5:78-84.
- Beklemishev W. Some problems of epidemiology and epizootology of tick-borne encephalitis [In Russian]. Med Parazitol (Moscow). 1959;3:310-8.
- Labuda M, Austyn J, Zuffova E, et al. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 1996;219.
- Rasnitsyn S. Evaluation of the importance of transphase and transovarial transmission for preservation of the causative agent population [In Russian]. Med Parazitol. 1976;3:269-74.
- Alekseev A. Ecology of tick-borne encephalitis virus: part of Ixodidae tick males in its circulation. Ecolog Parasitol.(Leningrad, Petrozavodsk). 1991;1:51-62, 100.
- Bakhvalova V, Potapova O, Panov V, Morozova O. Vertical transmission of tick-borne encephalitis virus between generations of adapted reservoir small rodents. Virus Res. 2009;140:172-8.
- Rosa R, Pugliese A. Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math Biosci. 2007;208:216-40.
- Bogovic P, Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases 2015;3:430-41.
- Kriz B, Benes C, Daniel M. Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997-2007). Epidemiol Mikrobiol Immunol. 2009;58:98-103.
- Hudopisk N, Korva M, Janet E, et al. Tick-borne encephalitis associated with consumption of raw goat milk, Slovenia. Emerg Infect Dis. 2013;19:806-8.
- Holzmann H, Aberle S, Stiasny K, et al. Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Emerg Infect Dis. 2009;15:1671-3.
- Helpert A, Sinnecker H. Ausgewählte Erhebungen zur Zeckenenzephalitis-Epidemie im Kreis Niesky, Bezirk Dresden. Dt Gesundh-Wes. 1966;21:1277-9.
- Kondrashov aZ, Filippovets R. Infection-rate of Ixodes persulcatus ticks and some aspects of transovarial transmission after their dosed infection with tick-borne encephalitis virus. Voprosy Virusol. 1970;6:703-8.
- Alekseev A, Burenkova L, Chunikhin S. Peculiarities of behaviour of ticks Ixodes persulcatus P.Sch., infected by tick-borne encephalitis virus [In Russian]. Med Parasitic Dis. 1988;2:71-5.
- Rizzoli A, Hauffe HC, Tagliapietra V, Neteler M, Rosa R. Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLoS One. 2009;4:e4336.
- Pretzmann G, Loew J, Radda A. Untersuchungen in einem Naturherd der Frühsommer-Meningoencephalitis (FSME) in Niederösterreich. Zentralbl Bakteriol Orig. 1964;194:431-9.
- Süss J. Tick-borne encephalitis in Europe and beyond – the epidemiological situation as of 2007. Euro Surveill. 2008;13:18916.
- Jaenson T, Hjertqvist M, Bergström T, Lundkvist Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors. 2012;5:184.
- Chunikhin S. Experimental investigation on tick-borne encephalitis virus ecology. Vopr Virusol. 1990;35:183-7.
- Sándor A, Mărcuțan D, D’Amico G, Gherman C, Dumitrache M, Mihalca A. Do the ticks of birds at an important migratory hotspot reflect the seasonal dynamics of Ixodes ricinus at the migration initiation site? A case study in the Danube Delta. PLoS One. 2014;9:e89378.
- Tonteri E, Jokelainen P, Matala J, Pusenius J, Vapalahti O. Serological evidence of tick-borne encephalitis virus infection in moose and deer in Finland: sentinels for virus circulation. Parasit Vectors. 2016;9:54.
- Korenberg E, Kovalevsky Y. General scheme of tick-borne encephalitis virus circulation. Zool Zhurnal. 1977;56:1467-78.
- Norman R, Bowers R, Begon M, Hudson P. Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. J Theoretical Biol. 1999;200:111-8
- Hartemink N, Randolph S, Davis S, Heesterbeek J. The basic reproduction number for complex disease systems: Defining R0 for tick-borne infections. Am Nat. 2008;171:743-54.
- Kožuch O, Grešíková M, Nosek J, Lichard M, Sekeyova M. The role of small rodents and hedgehogs in a natural focus of tick-borne encephalitis. Bull World Health Organ. 1967;36(Suppl 1):61.
- Alekseev A, Burenkova L, Vasilieva I, Dubinina H, Chunikhin S. Preliminary studies on virus and spirochete accumulation in the cement plug of ixodid ticks. Exp Appl Acarol. 1996;20:713-23.
- Demina TV, Dzhioev YP, Verkhozina MM, et al. Genotyping and characterization of the geographical distribution of tick-borne encephalitis virus variants with a set of molecular probes. J Med Virol. 2010;82:965-76.
- Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2011;2:2-15.
- Stefanoff P, Pfeffer M, Hellenbrand W, et al. Virus detection in questing ticks is not a sensitive indicator for risk assessment of tick-borne encephalitis in humans. Zoonoses Public Health. 2013;60:215-26.
- Katargina O, Russakova S, Geller J, et al. Detection and characterization of tick-borne encephalitis virus in Baltic Countries and Eastern Poland. PLoS ONE. 2013;8:e61374.
- Biernat B, Cieniuch S, Stańczak J. Detection of TBEV RNA in Ixodes ricinus ticks in north-eastern Poland. Ann Agr Env Med. 2014;21:689-92.
- Cuber P, Andreassen Å, Vainio K, et al. Risk of exposure to ticks (Ixodidae) and the prevalence of tick-borne encephalitis virus (TBEV) in ticks in Southern Poland. Ticks Tick Borne Dis. 2015;6:356-63.
- Valarcher J, Haglund S, Juremalm M, et al. Tick-borne encephalitis. Rev Sci Tech. 2015;34:453-66.
- Streissle G. Studies in the transmission of the virus of early summer meningoencephalitis by the tick Ixodes hexagonus Leach. Zentralbl Bakteriol. 1960;179:289-97.
- Novak-Chmura M, Siuda K. Ticks of Poland. Review of contemporary issues and latest research. Ann Parasitol. 2012;58:125-55.
- Bekleshova A, Terskikh I, Smirnov V. Arboviruses isolated from bird ticks Ceratoxides putus Pick.-Camb. Collected in areas in the extreme north [In Russian]. Vop Virus. 1970;15:436-40.
- Golovljova I, Vene S, Sjölander K, Vasilenko V, Plyusnin A, Lundkvist A. Characterization of tick-borne encephalitis virus from Estonia. J Med Virol. 2004;74:580-8.
- Takeda T, Ito T, Osada M, Takahashi K, Takashima I. Isolation of tick-borne encephalitis virus from wild rodents and a seroepizootiologic survey in Hokkaido, Japan. Am J Trop Med Hyg. 1999;60:287–91.
- Rieger M, Nübling M, Müller W, Hasselhorn H, Hofmann F. Foxes as indicators for TBE endemicity a comparative serological investigation. Zentralbl Bakteriol. 1999;289:610-8.
- Wurm R, Dobler G, Peters M, Kiessig S. Serological Investigations of red foxes (Vulpes vulpes L.) for determination of the spread of tick-borne encephalitis in North-Rhine-Westphalia. J Vet Med. 2000;47:503-9.
- Jemeršić L, Dežđek D, Brnić D, et al. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus elaphus) in Croatia. Ticks Tick Borne Dis. 2014;1:7-13.
- Gerth H, Grimshandl D, Stage B, Döller G, Kunz C. Roe deer as sentinels for endemicity of tick-borne encephalitis virus. Epidemiol Infect. 1995;115:355-65.
- Skarphédinsson S, Jensen P, Kristiansen K. Survey of tick- borne infections in Denmark. Emerg Infect Dis. 2005;11:1055-61.
- Rizzoli A, Neteler M, Rosa R, Versini W, Cristofolini A, Bregoli M, Buckley A, Gould EA. Early detection of tick-borne encephalitis virus spatial distribution and activity in the province of Trento, northern Italy. Geospat Health. 2007;1:169-176.
- Cisak E, Wójcik-Fatla A, Zajac V, Sroka J, Buczek A, J. D. Prevalence of tick-borne encephalitis virus [TBEV] in samples of raw milk taken randomly from cows, goats and sheep in Eastern Poland. Ann Agr Env Med. 2010;17:283-6.
- Balogh Z, Egyed L, Ferenczi E, Bán E, Szomor KN, Takács M, Berencsi G. Experimental infection of goats with tick-borne encephalitis virus and the possibilities to prevent virus transmission by raw goat milk. Intervirology. 2011;3:3:194-200.
- Takashima I, Morita K, Chiba M, Hayasaka D, Sato T, Takezawa C, Igarashi A, Kariwa H, Yoshimatsu K, Arikawa J, Hashimoto N. A case of tick-borne encephalitis in Japan and isolation of the virus. J Clin Microbiol. 1997;35:1943-1947.
- Lommano E, Dvořák C, Vallotton L, Jenni L, Gern L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 2014;6:871-82.
- Csank T, Bhide K, Bencúrová E, Dolinská S, Drzewnioková P, Major P, Korytár Ľ, Bocková E, Bhide M, Pistl J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch Virol. 2016;6:1679-83.