Key Points
- TBE can cause clinical symptomatic disease in dogs and horses
- Diagnosis of TBEV infection in animals is similar to diagnosis in humans
- Animals can be used as sentinels for human exposure
Introduction
While tick-borne encephalitis (TBE) is well documented as a public health threat, the veterinary aspects of this zoonotic disease are little noticed. TBE in animals has, for very long, been considered to be a problem exclusive to domestic ruminants due to their known potential to transmit tick-borne encephalitis virus (TBEV) via milk to consumers. While clusters of such cases continuously declined with the invention of milk pasteurization and overall improvements in hygiene management in cattle farming, goats and sheep flocks are still kept in traditional grazing farms where they are exposed to TBEV-infected ticks.1,2 In other words, even in industrialized countries, consumption of raw milk products continues to be a risk factor to acquire a TBE infection. As society continues to exhibit a trend towards a preference for natural products (assuming consumers can afford these), alimentary TBEV infections may be observed more frequently in the future (see chapter 7). While this is a ‘direct’ zoonotic aspect of TBE (besides the tick bite of course), animals play a role in TBEV transmission in many other ways; either as diseased dead-end hosts, as infected animals without obvious burden of disease, or in maintaining and spreading the virus itself or the TBEV- harboring tick.
In this chapter, we cover what is known in animal species (dogs and horses) that develop disease with strikingly similar clinical symptoms as humans. Then, we describe the animal species which readily become infected with TBEV, without developing any kind of illness, but which serve as a source of the infection for humans via the alimentary route (domestic ruminants). We then focus on other animal species that could be used either as sentinels for natural TBE foci: primarily game animals (such as cervids and wild boar), which provide easy access to sampling; or which are known to be reservoir hosts to the virus (small mammals). In particular, it is the population and infection dynamics of the latter that are suspected to be the main drivers of TBEV prevalence in ticks and, consequently, of human TBE incidence.
Table 1: Serosurveillance studies for TBEV and TBEV antibodies in dogs
| Year | Country | Number of dogs | Clinical signs | Virus detection | Reference | Results |
|---|---|---|---|---|---|---|
| 1988 - 1991 | Sweden | 255 | not observed | n.d. | 104 | 18 seropositive |
| 1993 - 1998 | Germany | ~ 1000 dogs | not observed | n.d. | 105 | 2% - 31% ELISA seropositive |
| 1994 - 1995 | Japan | 10 sentinel dogs each year | not observed | 3 virus isolates | 106 | high Ab-titers upon seroconversion ELISA & SNT |
| 1997 - 1998 | Czech Republic | 151 dogs | in 3 dogs | n.d. | 10 | HIT |
| 1999 | Austria | 552 dogs | in 57 seropositive dogs | n.d. | 14 | 133 seropositive (24.1%, ELISA), 110 confirmed by SNT (19.9%) |
| 1998 - 2003 | Norway | 317 dogs | not observed | n.d. | 12 | 52 seropositive (16.4%) 2 different ELISA |
| 2002 | Germany | 54 healthy and 56 ill dogs | in 56 dogs, not further specified | n.d. | 7 | 17/54 positive |
| 30/56 positive | ||||||
| 2005 - 2006 | Denmark | 125 dogs | not observed | n.d. | 9 | 30% ELISA, 4.8% SNT |
| 2009 | Belgium | 960 dogs | not observed | n.d. | 8 | 0.1% positive (ELISA, HIT & SNT) |
| 2011 | Austria | 90 dogs | not observed | n.d. | 3 | repeated testing within one year: 9.8% - 13.4% seropositive (ELISA) |
| 2011 - 2012 | Czech Republic | 159 dogs | in 7/20 viremic dogs | by PCR | 11 | 11.3% seropositive dogs, viremic dogs 12.6% (ELISA) |
| 2011 - 2012 | Finland | 148 dogs | not observed | n.d. | 13 | 6% - 40% seropositive (2 ITF & 2 ELISA) |
| 2012 - 2014 | Germany | 331 healthy dogs | not observed | n.d. | 6 | 2.1% seropositive dogs (ELISA and SNT) |
| 2013 - 2015 | Spain | 815 healthy dogs | not observed | n.d. | 114 | 1.7% seropositive dogs (ELISA and SNT) |
Dogs
Canine TBEV infection is a frequent event in endemic areas, with a calculated annual risk of about 11.6%.3 Total seroprevalence in the canine population has been examined in several countries: Switzerland 3.6–5.9%,4 Greece 1–8%,5 Germany 2.1– 42.7%,6,7 Belgium 0.1%,8 Denmark 4.8–30%,9 Czech Republic 3.3–11.3%,10,11 Norway 16.4%,12 Finland 6–40%,13 and Austria 13.3–24%.3,14 As inclusion criteria were different regarding the presence of clinical symptoms, residence, and tick-exposure of the examined dogs, results are difficult to compare (Table 1). Different test systems (enzyme-linked immunosorbent assay [ELISA], serum neutralization test [SNT]) used in these studies clearly influenced the results too. TBE has always been stated to be a tick-borne infection, mainly transmitted by ixodid ticks; however, Dermacentor reticulatus ticks may play an important role in transmission to dogs.15 There has been one single case of a dog from the Czech Republic with a TBE-infection suspected to be due to consumption of raw goat milk.10
Figure 1

Figure 2
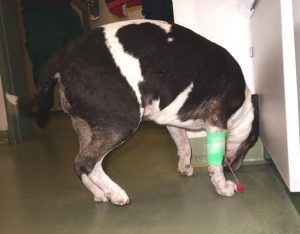
Figure 3
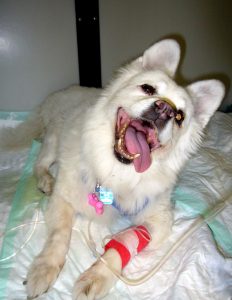
Course of disease
Despite frequent TBE infection in dogs, most dogs do not develop any clinical signs. Dogs seem to be less susceptible than humans, although a lethal outcome within the first week of disease is documented in 16–50% of clinically symptomatic cases in dogs. Infection may lead to an acute course of the disease, with complete remission of symptoms within 1–2 weeks (31–59%). Infrequently, prolonged disease courses are described with long time period to remission (12–25%). These dogs frequently suffer from late sequela–like paresis, muscle atrophy, epileptic seizures, or blindness (Figure 1).10,16,17
Clinical pictures
After an estimated incubation period of 5–9 days, first clinical symptoms occur and develop to a maximum level within 48 hours. Initially, most dogs are depressed and show non-specific signs such as salivation and vomiting (25%), refusal to eat, and are reluctant to move due to generalized weakness, although some dogs show compulsive walking, circling to one side (25%), unusual behavior (70–91%), and head pressing (Figure 2).10,16-19
The elevated body temperature (42–66%) may initially be classed as fever; later on it is more likely a result of non-voluntary excessive muscle contraction (e.g., seizures, loss of inhibition by upper motor neuron damage). Seizures are a principal result of cerebral damage due to TBEV infection and are observed in 12–33% of canine cases.17,19
Neurological symptoms like paresis (8–38%), vocalization due to painful perception of active and passive back movement (21–66%), and deficits of the cranial nerves (16–50%) (Figure 3) develop within a few hours thereafter.17,19,20
Blindness due to papillitis, optic nerve inflammation, or chiasma opticus neuritis may become the dominant symptom and systemic signs may diminish. Visual deficits may be the major clinical sign of disease and result from detachment of peripapillary retina, peripapillary hemorrhages, and inflammatory edema.21,22 Degeneration and demyelination of cranial nerves is certainly initiated by the virus’ neurotropism. Later on, secondary immune reaction to neural tissue may prolong the period of damage and lead to irreversible symptoms such as retinal and optic disc atrophy. Other cranial nerve deficits like trigeminal dysfunction, resulting in reduced facial sensation and chewing muscle atrophy, vestibular signs (nystagmus and positional strabismus, Figure 4), and facial palsy, are observed.
Brainstem symptoms like arhythmical breathing pattern may be present in comatose dogs, especially in severe cases with guarded prognosis (in TBE Book online version)
Major involvement of the spinal cord results in mostly symmetrical paresis, muscle twitching, and proprioceptive dysfunction (38-50%), which may also be present as an exclusive symptom and may occur asymmetrically (Figure 5).10,17,19,20
There is no significant breed, gender, or age predisposition, although most cases are described in adult middle- to large-breed dogs. Rottweilers and Huskies are overrepresented in the literature14,20,21 (Table 2).
Figure 4

Major involvement of the spinal cord results in mostly symmetrical paresis, muscle twitching, and proprioceptive dysfunction (38-50%), which may also be present as an exclusive symptom and may occur asymmetrically (Figure 5).10,17,19,20
Figure 5

There is no significant breed, gender, or age predisposition, although most cases are described in adult middle- to large-breed dogs. Rottweilers and Huskies are overrepresented in the literature.14,20,21 (Table 2).
Brainstem symptoms like arrhythmical breathing pattern may be present in comatose dogs, especially in severe cases with guarded prognosis
Video: Comatose dog of Figure 4 with arhythmical breathing indicative of brain stem lesion
Major involvement of the spinal cord results in mostly symmetrical paresis, muscle twitching, and proprioceptive dysfunction (38-50%), which may also be present as an exclusive symptom and may occur asymmetrically (Figure 5).10,17,19,20
There is no significant breed, gender, or age predisposition, although most cases are described in adult middle- to large-breed dogs. Rottweilers and Huskies are over-represented in the literature14,20,21(Table 2).
Laboratory findings and diagnosis
A definite diagnosis in dogs with TBE is rarely achieved intra vitam as it has to be supposed to be very unlikely to detect the virus in the blood or in the cerebrospinal fluid (CSF). In 1 study from the Czech Republic, 12.6% of canine blood samples tested positive for TBEV by nested RT-polymerase chain reaction (PCR), although only one-third of these dogs suffered from neurological symptoms.11
Whether the other dogs were in an asymptomatic carrier status, or just happened to be tested during their incubation period, as reported in humans, remained unclear. Virus detection in the CSF has been achieved only in isolated cases within the first 3 days of disease.19 Immunological rapid virus clearance in the dog’s brain and CSF seems to be very fast and completed before most diagnostic procedures are performed. The inability of the central nervous system’s (CNS) local immune system to eliminate the virus within a few days is probably the reason for a fatal outcome, as in most of these cases no specific intrathecal antibody production and no increased cell count in the CSF were detected prior to death.17
Table 2: Case reports and case series of TBE in dogs
| Year | Country | Dog breed | Clinical symptoms | Reference | Antigen detection | Antibody response | Confirmation | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1960 | Sweden | 1 dog | n.d. | 107 | n.d. | yes | Antibody response | |
| 1970 | Switzerland | 1 Landseer | behavioural changes, fever, tremor, paresis, seizures | 108 | yes | n.d. | Virus isolation | died |
| 1993 | Switzerland | 2 Rottweiler, 1 Greyhound, 1 Husky, 1 Golden Retriever | ataxie, tetraparesis, fever, grand mal seizures | 20 | n.d. | IgM in CSF in 2 dogs | IHC | all 5 dogs were euthanized |
| 1994 -1997 | Austria | 3 Husky, | tremor,ataxia, hyperesthesia, hem-tetraplegia, opisthotonus, anisocoria, miosis, nystagmus | 25 | n.d. | n.d. | IHC, pathohistological changes | all 8 dogs died or were euthanized |
| 1 Terrier-Mix, 1 Rottweiler, | ||||||||
| 1 Irish Setter, 1 Bastard, | ||||||||
| 1 Pekingese | ||||||||
| 1998 | Germany | 1 Rottweiler, | fever, hyperesthesia, seizures, opisthotonus, facial nerve paresis, strabismus | 109 | n.d. | Yes (both dogs) | Antibody response | one fully recovered, one partially recovered |
| 1 Newfoundland dog | ||||||||
| 1996-1998 | Germany | 2 Rottweiler, 1 Newfoundland dog *, 1 Boxer | ataxia, fever, weakness, tetraparesis, cranial nerve deficits, seizures | 18 | n.d. | yes | Antibody response (SNT in 2 dogs) | 2 euthanized, 2 fully recovered |
| 2001 | Czech Republic | 3 Rottweiler, 1 Fila Brasiliero, 1 Dachshound | behavioural changes, ataxia, tetraplegia, hyperesthesia, | 10 | n.d. | yes | Antibody response | 1 asymptomatic, 1 partially recovered, 3 fully recovered |
| 2001-2002 | Sweden | 1 Riesen-schnauzer | behavioural changes, fever, ataxia, tretraplegia | 110 | n.d. | yes | Antibody response | |
| 2006 | Sweden | 1 dog | ataxia, tremor | 111 | n.d. | yes | Antibody response | |
| 2007 | Sweden | 2 dogs | Fever, ataxie, tremor, hyperesthesia | 112 | n.d. | yes | Antibody response | full recovery after one year |
| 2008 | Austria | 8 dogs (including 1 Rottweiler, 2 Husky) | Ataxia, grand mal seizures, hyperesthesia, fever, compulsive walking, blindness | 17 | negative from csf in 2 dogs | yes | 2 IHC, 6 antibody response | 2 died, 6 fully recovered |
| 2009 | Italy (imported from Hungary) | 1 bastard | ataxia, weakness, hyperesthesia | 113 | n.d. | n.d. | PCR and IHC | euthanized |
| 2011-2012 | Czech Republic | 7 dogs | seizures, disorientation, central vestibular syndrome, paraparesis, cranial nerve deficits | 11 | yes, PCR from blood | yes | Virus detection, Antibody response | |
| 2012-2014 | Switzerland | 12 dogs (including 2 Labrador, 1 Rottweiler, 1 Husky, 1 Newfoundland dog) | behavioral changes, ataxia, seizures, paresis, cranial nerve deficits, hyperesthesia | 24 | n.d. | Yes in 11 dogs | Antibody response, IHC in 5 dogs | 6 euthanized, 6 fully recovered |
*1 dog was also published in a previous paper CSF = cerebrospinal fluid; Ig = immunoglobulin; IHC = immunohistochemistry, n.d. = not determined; PCR = polymerase chain reaction; SNT = serum neutralization test
CSF analysis in affected dogs with clinical signs mostly reveals elevated leukocyte count, with predominantly mononuclear cells and elevated total protein. CSF changes are concomitant to virus elimination and rising antibody titers. Specific antibodies are detectable in the serum of affected dogs within a few days.7,17,18,20 Cross-reactivity to Louping ill virus, West Nile virus, and Usutu virus should be taken into consideration in endemic areas.10,23 Magnetic resonance imaging findings included bilateral and symmetrical gray matter lesions involving the thalamus, hippocampus, brain stem, basal nuclei, and ventral horn on the spinal cord.
All lesions had minimal or no mass effect, or perilesional edema.24 These findings are comparable to the distribution of lesions in the canine brain detected by necropsy and immunohistochemistry.25
Proton magnetic resonance spectroscopy, to evaluate metabolic abnormalities in dogs with TBE, revealed significant differences with dogs with immune mediated meningoencephalitis and healthy dogs.26
A tentative diagnosis of TBE in dogs should fulfill the following criteria: tick exposure or observed tick infestation, neurological signs indicative for a diffuse or multifocal CNS disease, (mostly mononuclear) pleocytosis in the CSF, a positive antibody titer in serum or CSF, or in the case of fatal outcome a positive virus confirmation within the brain or spinal cord. In the future, highly sensitive PCR techniques may include virus detection in the diagnostic work-up in early stages of disease. Increasing serum titers may be detected, but more often rapidly decreasing titers are observed when dogs reach partial or complete remission of clinical signs.17,26
Possible differential diagnoses include other viral meningoencephalitis such as distemper, rabies, pseudorabies, as well as protozoal, bacterial, or fungal meningoencephalitis, and paraneoplastic and immune mediated meningoencephalitis.
Figure 6

Treatment
Symptomatic therapy is strongly recommended for dogs with TBE. Water and food maintenance orally, by constant rate infusion, or by gastric tubes and supportive care is essential. Sedation and relaxation are necessary in the case of seizures. Steroid use is controversial, as immunosuppression may prolong the presence of the virus. In dogs with marked CSF pleocytosis, steroids seem to be mandatory to effectively protect the brain tissue from further fulminant immune response. In cases of muscle atrophy and paresis, physiotherapy (Figure 6) as early as possible has been shown to improve the general outcome and shorten the time of rehabilitation.19,20
Prevention
There is no licensed anti-TBE vaccine for dogs, although dogs develop detectable antibody titers after vaccination with a human vaccine.27 Tick protection is the most important measure to avoid transmission and infection, mainly performed by regular administration of acaricidal substances (spot on, tablets, shampoos, collars) and immediate tick removal after detection by the owner.3
Regular anti-tick measures are essential to reduce transmission risk all through the year as single canine cases have been reported even during the cold seasons of the year.21
Horses
Although the first clinical case of laboratory-confirmed TBE in a horse was published more than 35 years ago,28 our knowledge about the impact of TBEV in horse populations is scarce. To the best of our knowledge, there are 4 published studies where clinical signs of neurological disorder could be traced to the TBEV as etiology. After the aforementioned initial published case from Switzerland, 8 horses with clinical symptoms were described in Austria, 2 of which were severely ill;29 1 out of 3 diseased animals from a study in Germany had to be euthanized;30 and again in Germany, some years later, an infected animal had to be euthanized.31
The clinical picture in horses mirrors that which we described for dogs, displaying a broad spectrum of neurological symptoms: ataxia, tonic-clonic seizures, apathy and stupor, inappetence, mydriasis, convulsions of the legs, skittishness, bruxism, and altered reactions to environmental stimuli. Regarding therapeutic options and prognosis, a horse with recumbent status due to TBE has a poor prognosis as long as it is not possible to force the horse to stand up again.
The few case reports available suggest that clinical TBE in horses is a rare event, although basic horse population-based data are missing. Looking at the few seroprevalence studies in horses, the prevalence of anti-TBE-antibodies ranged from 26.1% and 13% in Austria29,32 to 2.9% in central Germany,30 and 5.2% and 23.4% in southern Germany31,33 to 0 of 40 horses investigated in Hungary34 or 0 of 2349 horses from the Czech Republic.35 Cross-reactivity to other flavivirus may influence these results.35,36 Horses have been suggested to be good sentinel animals for human TBEV infection risk, because they readily seroconvert upon infection, but they stick more to a given territory in comparison to dogs who, as family members, travel more.
Domestic ruminants
For more than half a century, grazing cattle, goats, and sheep have been known to be susceptible to TBEV infection. Interestingly, these ruminants do not develop any clinical symptoms, and even after experimental infection, a slight elevation of body temperature is a rare finding.37,38 However, in 2015, a five-month-old lamb in Bavaria dis-played symptoms of a neurological disorder, and after euthanasia, TBEV infection was subsequently diagnosed.39 Whether this case was the result of an unknown underlying disease or immunosuppressive factors cannot be determined. TBE in domestic ruminants, if it occurs at all, appears to be an extreme exception. Nevertheless, infected animals develop viremia with a duration of up to 19 days.40 A study in the Swiss canton of Valais found 4.25% of the tested goats to be seropositive according to an ELISA test, with 40.4% of these testing positive on a serum neutralization test (SNT).41In the canton of Ticino, with no history of TBE, SNT-positive goats were found in 10 out 37 flocks (14.6% out of 662 sera).42
Even if the viremia is shorter than 1 week, the virus is shed via milk and remains infectious in cheese or other products prepared from unpasteurized milk. Consumption of such products may have led to an alimentary infection of a group of individuals who became infected through the same batch of contaminated food, resulting in clusters of human cases.43 Such clusters of cases have recently been reviewed2 and were thought to be restricted to nations in Eastern Europe with Slovakia having the highest occurrence of alimentary TBE outbreaks in Europe.44 However, alimentary TBEV infection with clinical TBE occurred recently in Germany as a result of consumption of fresh raw goat milk45. As there is a growing trend towards consumption of natural food products in the industrialized nations of Western Europe, such scenarios may be witnessed more frequently in the future. One study in an endemic region in Poland found TBEV in milk from sheep (22.2%), goats (14.8%), and cows (11.1%).46 In Norway, a study found TBEV RNA in 5,4% of tested raw milk samples. Positive blood serum samples only occurred in one municipality, where 88.2% of tested cows had specific antibodies. Remarkably, none of the cows with a positive milk sample had detectable antibodies and vice versa.47 Domestic ruminants do develop an antibody response, wich in the case of goats and sheep is measurable for at least 28 months or even up to 6 years and 10 months.23,27,48 Exposure to TBEV seems not to result uniformly in seroconversion of the entire flock of animals.49,50 Whether this indicates that not all animals of the same herd were exposed and infected or that some animals did not mount an immune response is not known. Also, the extent of antibody response seems to vary between the species.51
Game animals (wild boar, cervids, foxes)
Roe deer (Capreolus capreolus) are the most abundant cervids in Germany, sharing their habitat with ticks everywhere. They are well known as hosts for nymphs and adult ticks and thus are as important to maintenance of the tick population as the small mammals are for larvae and nymphs (see below). It is common to find hundreds of ticks per individual and, consequently, the odds of roe deer becoming infected in TBE-endemic areas are rather high.52 Therefore, they can be a useful tool to identify endemic areas as could be seen in the Netherlands, where TBE was regarded as an imported disease until 2016. Serologic screening there showed TBEV-neutralizing antibodies with a seroprevalence of 2% in roe deer.53 However, clinical or pathological signs that raise suspicions of an overt TBEV infection have never been described for roe deer. Seroconversion after infection seems to be the rule, and this fact has been widely used to estimate TBE prevalence in certain areas. As roe deer are territorial animals, many researchers claim that this serological data could be very useful in finding and describing possible TBE-endemic areas, in particular in low-endemic areas or regions in which TBE cases in humans are reported only sporadically.54-61The discrepancy of often double-digit percentages of seroprevalence in roe deer and no, or almost no, human cases is puzzling, and needs to be investigated further. As TBEV is known to be circulating in such areas, an understanding of why only few or no human cases occur could be key to developing strategies aimed at reducing TBE incidence in high-endemic areas (as defined by the number of human cases).
Likewise, the wild boar (Sus scrofa) is present all over Europe and is commonly infested with ticks. There are no records of a possible TBE-like disease in wild boar and only 2 studies investigated the seroprevalence against TBEV in wild boar. Nevertheless, these studies demonstrated a surprisingly high percentage of animals with antibodies against TBEV in areas with no notified human TBE cases.59 A sero-survey of wild boar in Belgium revealed the presence of TBEV, with 2.9% of the 238 wild boar investigated having specific neutralizing antibodies against TBEV.62 As Belgium is considered to be traditionally free of autochthonous TBE,2,63,64 this study demonstrates the power of using animal surveillance data for pinpointing TBE-endemic areas. A similar approach was applied in France using wild boar and roe deer sera with similar results, i.e. 2.9% and 0.3% seropositive animals.65 Like the roe deer described above, wild boars are rather territorial, allowing the geographical allocation of such data. Only the renegade wild sows are known to travel across large areas when they are searching for a new herd. A study from the Czech Republic, traditionally a country with a high TBE incidence, found a positive association between the number of hunted wild boar and human cases. Consequently, the authors concluded that wild boar must play a role in TBEV transmission either directly or indirectly.66
In Finland, moose (Alces alces) and white-tailed deer (Odocoileus virginianus) were found to harbor TBEV-specific antibodies (0.74%) and the use of such seroprevalence data as an indicator for local risk of human TBE infection is recommended.67 In Norway 9.4% of 286 moose, 1.4% in red deer and 0.7% in roe deer led to an overall seroprevalence of 4.6% in cervids. Interestingly none of the 83 investigated reindeer showed antibodies against TBEV.68 One single case report describes the pathological and immunohistological findings in a mouflon (Ovis ammon musimon) with marked encephalitis due to TBEV.69 A Polish study analyzed D. reticulatus collected from the lowland European bison (Bison bonasus bonasus) in a known endemic focus and found 18.42% of these ticks to be positive for TBEV RNA.70 In Japan, the seroprevalence in raccoons varied between 0.8% and 5.9% in eastern and central Hokkaido province while sika deer (Cervus Nippon) showed in TBEV-neutralizing antibodies in 0.8% and 2.4% there.71 Interestingly, not much is known about the role of foxes (Vulpes vulpes) in natural TBE foci. Although it is a highly prevalent predator of small mammals (see below), and is regularly infested with Ixodes ticks, there are no recent studies investigating virus or antibodies against TBEV in foxes. Older studies from Germany were mostly performed in non-endemic areas on the German-Dutch border and Brandenburg, and consequently revealed no seroprevalence or a single sero-reactive serum sample only.72,73 However, the latter report found every third fox in South-Western Germany to have antibodies against TBEV.73 In Croatia, a study found at least 1.6% of ticks on red foxes and 1.1% of spleen samples of red deer (Cervus elaphus) to be positive for TBEV-RNA.74 It would be interesting and necessary to perform a seroprevalence study in a known endemic area to shed light on the role of the fox in the natural transmission cycle of TBEV and to prove the putative positive correlation between fox abundance and TBE incidence.73,75
Studies trying to detect a correlation between human TBE incidence and abundance of certain animals are contradictory. A Swedish study revealed that, with one year of time-lag, the abundance of roe deer, red deer, mountain hare (Lepus timidus) and European hare (Lepus europaeus) showed positive covariance with the incidence of human TBE. In contrast, moose and fallow deer (Dama dama) showed negative covariance and wild boar, lynx (Lynx lynx) and red fox showed no significant covariance with human TBE incidence.69 In Slovenia, red deer abundance was correlated with human TBE incidence when including a three-year time-lag, whereas roe deer showed no significant correlation.77 An Italian study found roe deer density to have a better predictive value for a model explaining the increasing human TBE incidence than red deer density.78
Small mammals
Small mammals have an essential role in the maintenance of TBE foci in 2 ways. Firstly, rodents and, to a lesser extent, shrews are the main hosts for Ixodes larvae. Without this first blood meal, a tick population would die out over time. They are also hosts for nymphs when they take their blood meal, which is needed before they can molt into adult ticks. Secondly, they are reservoir hosts for TBEV and thus responsible for re-infections of ticks via transovarial transmission, i.e., the transfer of TBEV from a female tick to her eggs, although this is negligible for the epidemiology of the virus. The reservoir function, however, has large implications for the longevity of a natural focus. As outlined earlier, in the chapter on transmission and natural cycle, infection of a tick can occure via a viremic host, but another phenomenon has been described which also applies to the infection of ticks while feeding on small mammals. The so-called co-feeding allows the infection of Ixodes larvae when an infected Ixodes nymph feeds in close proximity. In this case, the rodent does not have to be infected, because the virus finds its way from the nymph directly to the larva.79 So, it is safe to say that, in many ways, rodents are as necessary as Ixodes ticks for maintaining the TBEV life-cycle. In particular bank voles (Myodes glareolus) appear to be well adapted to TBEV, leading to long-lasting viremias and infiltration of the brain without causing visible neurological symptoms.80
Recent publications have reviewed the prevalence of either viral ribonucleic acid (RNA) or specific antibodies against TBEV in rodents in various countries.81-83 The antibody prevalence in endemic areas was found to range between 0% and 5.9%. However, seroprevalence rates up to 12.5% were found in some rodent species (e.g., the bank vole, Myodes glareolus),84 suggesting a differing role of particular rodent species in a TBE focus. Viral RNA can also be found in wild rodents, with an even higher prevalence of up to 15%.85 Studies from Hungary identified TBEV-RNA in 4.2%86 and TBEV-specific anti-bodies in 5.19% and 4.93% of the tested small rodents.87 Recently, TBEV-positive bank voles (and ticks) were found in a forest within the city borders of Moscow, Russia.88 Experimentally infected common voles (Microtus arvalis) harbored infectious TBEV for at least 3 months.85 Viral RNA could be found in the brain tissue of experimentally infected bank voles for up to 168 days.89 This has important implications, as the brain (and to a lesser extent other organs such as kidney and spleen) seems to be the prime site of virus persistence in rodents. Indeed, TBE viral RNA was found in the brain tissue of naturally infected field voles (Microtus agrestis) and bank voles in Finland, after the winter but before the tick season started.90 Seroprevalence in Microtus rodents were found to be 4% in Poland.91 Thus rodents seem, along with transstadially-infected ticks, to play a role in the ‘overwintering’ of the TBEV.
Other mammals and birds
As most animals do not develop overt disease upon infection with TBEV, many mammal species have never been investigated as to whether or not they are susceptible to an infection or capable of developing an immune response in terms of measurable antibody titers. According to the broad geographic distribution of TBE covering most of Europe and northern Asia, we consider that there may be many mammal species not yet investigated that react to an infection in a similar manner as described above for wild boars or roe deer, i.e., seroconversion without clinical disease. One exception is the Barbary macaque (Macaca sylvanus), a monkey species not native to Eurasia, despite a small population in Gibraltar, the southernmost tip of Spain. An individual of a small group of these animals kept in southwest Germany in an outdoor area fell severely ill with central nervous symptoms and was euthanized for ethical reasons. A pan-encephalitis was diagnosed and TBEV was demonstrated by immunohistochemistry, real-time RT-PCR, and virus isolation.92,93 Other individuals of this monkey group sero-converted without showing clinical signs.94 Thus far, we are not aware of further case reports of non-native species kept in semi-free holdings or zoos.
Birds are known to be readily infested with ixodid ticks and are prime suspects for long-distance transportation of ticks.95 The first studies investigating the prevalence of TBEV-harboring ticks on birds came from the Ottenby Bird Observatory at the southern tip of the island Öland in Sweden. During the annual ringing, more than 1000 Ixodes spp. ticks were collected from birds, with 0.52% showing TBEV RNA.96 Subsequent studies from Estonia (0.4% positive nymphs97), Switzerland (0.27% TBE viral RNA positive98), Latvia (14%99), Germany (no TBE virus found in almost 2500 Ixodes ricinus ticks collected from birds84) and Slovakia100 (a brain sample in a buzzard, Buteo buteo) demonstrated the possibility that TBEV can be transported over rather long distances via infected ticks attached to birds.
Studies from the 1960s failed to demonstrate both viremia and clinical illness in great tits (Parus major), pheasants (Phasianus colchicus), falcons (Falco tinnunculus), and buzzards (Buteo buteo51). Only a small fraction of infected animals seroconverted. Other birds, such as the house sparrow (Passer domesticus), common redpoll (Acanthis flammea), quail (Coturnix coturnix), and duck (Anas platyrhynchos), showed either detectable virus or even moderate viremia after infection.101 Another study demonstrated that the presence of TBEV seems to vary according to season and bird species. Prevalence rates above 50% indicate that particular bird species like fieldfares (Turdus pilaris), bramblings (Fringilla montifrigilla), and the common redstart (Phoenicurus phoenicurus) may well play a role as a reservoir, or at least amplifying host, for TBEV.102
Veterinary diagnostic aspects
In general, the same diagnostic tests and methods are applied for animals as those that are currently in use for diagnostic purposes in humans (see Chapter 10: Diagnosis). With the exception of diseased dogs and horses, which are usually under tight supervision by their owner, the time window to use any direct detection method for TBEV – isolation or real-time RT-PCR – is usually too short to be of any practical relevance. Immuno-histochemistry may be used in euthanized animals. In epidemiological studies using rodents, these methods may be applied as virus and viral RNA can be detected in the brain tissue of infected animals for months (see above). In contrast, serology can be easily applied in any animal species. Three test formats are frequently used for this purpose, i.e., ELISA, IFA (immunofluorescence assay), and SNT. The ELISA can be performed with a species-specific conjugate, which is available for dogs, cattle, sheep, goats, swine (works also for wild boar), cervids, and mouse (works also for voles and mice). However, there is a commercially available, species-independent ELISA which uses protein G-coupled enzyme. Although this test is also available for immunoglobulin (Ig)M antibodies, the IgG version should be used because of the reasons mentioned above. The IFA usually uses a mixture of uninfected and TBEV-infected Vero cells fixed on slides and the antibody-conjugates described for the ELISA. Finally, the SNT is the gold standard and is needed in order to verify results of the other 2 assays. According to the European Centre for Disease Prevention and Control, an SNT titer =1:10 confirms the diagnosis.23,27,103
Concluding remarks
Infections of various animals with TBEV are common in TBE-endemic areas, although they are barely noticed due to the lack of overt disease. The known exceptions are dogs and horses, which can become severely ill with the same panel of clinical symptoms, as the same neurological regions in the CNS are affected. Domestic ruminants are a risk for human health as they can shed TBEV through their milk for many days. If unpasteurized, TBEV-contaminated milk or milk products are ingested by consumers, and clusters of human cases may be the consequence. Many wild animal species become infected and develop an antibody response, but they do not appear to be harmed. Future research may address the potential use of antibody prevalence rates of particular animal species in order to complement the current risk definition for human infections, which at the moment is largely based on the count of human cases alone. Finally, while birds seem to play a role in long-distance transportation of TBEV-infected ticks and thus the geographic spread, small mammals, in particular rodents, are the key players in maintaining a TBE focus in nature.
Contact:
pfeffer@vetmed.uni-leipzig.de
Citation:
Pfeffer M, Schmuck HM, Leschnik M. TBE in animals. Chapter 8. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi: 10.33442/26613980_8-6
References
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861-71.
- Dobler G, Gniel D, Petermann R, Pfeffer M. Epidemiology and distribution of tick-borne encephalitis. Wien Med Wochenschr. 2012;162:230-8.
- Leschnik M, Feiler A, Duscher G, Joachim A. Effect of owner- controlled acaricidal treatment on tick infestation and immune response to tick-borne pathogens in naturally infested dogs from Eastern Austria. Parasit Vectors. 2013;6:62.
- Matile H, Ferrari E, Aeschlimann, A, Wyler R. Die Verbreitung der Zecken-enzephalitis in der Schweiz. Schweiz Med Wochenschr. 1981;111:1262-9.
- Chambouris R, Sixl W, Stunzer D, Köck M. Antibodies in dogs to the virus of tick-borne encephalitis (early summer encephalomyelitis/tick-borne encephalitis) in Greece. Geogr Med. 1989;3:11-14.
- Balling A, Beer M, Gniel D, Pfeffer M. Prevalence of antibodies against tick-borne encephalitis virus in dogs from Saxony, Germany. Berl Münch Tierärztl Wochenschr. 2015;128:297- 303.
- Reiner B, Grasmück S, Steffen F, et al. Prevalence of TBE antibodies in serum and CSF of dogs with inflammatory and non-inflammatory CNS disease. Int J Med Microbiol. 2002;291 (Suppl. 33):234.
- Roelandt S, Heyman P, Filette MD, et al. Tick-Borne Encephalitis Virus Seropositive Dog Detected in Belgium: Screening of the Canine Population as Sentinels for Public Health. Vector Borne Zoonotic Dis. 2011;11:1371-6.
- Lindhe KES, Meldgaard DS, Jensen PM, Houser GA, Berendt M. Prevalence of tick-borne encephalitis virus antibodies in dogs from Denmark. Acta Vet Scand. 2009;51:56.
- Klimeš J, Juřicová Z, Literák I, Schánilec P, Trachta e Silva E. Prevalence of antibodies to tick-borne encephalitis and West Nile flaviviruses and the clinical signs of tick-borne encephalitis in dogs in the Czech Republic. Vet Rec. 2001;148:17-20.
- Hekrlová A, Kubíček O, Lány P, Rosenbergová K, Schánilec P. Tick-borne encephalitis in dogs: application of ‘nested real- time RT-PCR’ for intraviral virus detection. Berl Münch Tierärztl Wochenschr. 2015;128:397-401.
- Csángó PA, Blakstad E, Kirtz GC, Pedersen JE, Czettel B. Tick- borne Encephalitis in Southern Norway. Emerg Infect Dis. 2004;10:533-4.
- Levanov L, Perez Vera C, Vapalahti O. Prevalence estimation of tick-borne encephalitis virus (TBEV) antibodies in dogs from Finland using novel dog anti-TBVE IgG Mab-capture and IgG immunofluorescence assays based on recombinant TBEV subviral particles. Ticks Tick Borne Dis. 2016;7:979-82.
- Kirtz G, Kölbl S, Czettel B, Thalhammer JG Frühsommer- Meningoenzephalitis (FSME, Zetraleuropäische Zeckenenzephalitis) beim Hund in Österreich: eine Seroprävalenz-Studie. Kleintierpraxis. 2003;48:133-40.
- Wójcik-Fatla A, Cisak E, Zajac V, Zwoliński J, Dutkiewicz J. Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick Borne Dis. 2011;2:16-19.
- Leschnik MW, Kirtz GC, Thalhammer JG. Tick-borne encephalitis (TBE) in dogs. Int J Med Microbiol. 2002;291(Suppl 33):66-9.
- Leschnik MW, Benetka V, Url A, et al. Virale Enzephalitiden beim Hund in Österreich: diagnostische und epidemiologische Aspekte. Vet Med Austria / Wien Tierärztl Monatsschr. 2008;95:190-9.
- Reiner B, Fischer A, Gödde T, Müller W. Clinical diagnosis of canine tick-borne encephalitis (tbe): Contribution of cerebrospinal fluid analysis (csf) and csf antibody titers. Zent Bl Bakteriol. 1999;289:605-9.
- Leschnik M. Tick-borne encephalitis in dogs. Proceedings of the Abildgaard Symposium, Copenhagen, Denmark. 2005:43-5.
- Tipold A, Fatzer R, Holzmann H. Zentraleuropäische Zeckenencephalitis beim Hund. Kleintierpraxis. 1993;38:619- 28.
- Stadtbäumer K, Leschnik MW, Nell B. Tick-borne encephalitis virus as a possible etiologic agent for optic neuritis in a dog. Vet Ophthal. 2004;7:271-7.
- Nell B. Optic Neuritis in Dogs and Cats. Vet Clin Small Anim. 2008;38:403-15.
- Klaus C, Ziegler U, Kalthoff D, Hoffmann B, Beer M. Tick-borne encephalitis virus (TBEV) – findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet Res. 2014;10:78.
- Beckmann K, Steffen F, Ohlerth S, Kircher PR, Carrera I. Three tesla magnetic resonance imaging findings in 12 cases of canine central European tick-borne meningoencephalomyelitis. Vet Radiol Ultrasound. 2016;57:41-8.
- Weissenböck H, Suchy A, Holzmann H. Tick-borne encephalitis in dogs: neuropathological findings and distribution of antigen. Acta Neuropathol. 1998;95: 361-6.
- Sievert C, Hening R, Beckmann K, Kircher PR, Carrera I. Comparison between proton magnetic resonance spectroscopy findings in dogs with tick-borne encephalitis and clinically normal dogs. Vet Radiol Ultrasound. 2016;58:53-61.
- Klaus C, Beer M, Saier R, Schubert H, Bischoff S, Süss J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl Münch Tierärztl Wochenschr. 2011;124:443-9.
- Waldvogel K, Matile H, Wegmann C, Wyler R, Kunz C. Zeckenenzephalitis beim Pferd. Schweiz Arch Tierheilkd. 1981;123:227-33.
- Luckschander N, Kölbl S, Enzensberger O, Zipko HT, Thalhammer JG. Tick-borne encephalitis (TBE) in an Austrian horse population. Tierärztl Prax Ausgabe G. 1999;27:235-8.
- Müller K, König M, Thiel HJ. [Tick-borne encephalitis (TBE) with special emphasis on infection in horses]. Dtsch Tierärztl Wochenschr. 2006;113:147-51 [in German].
- Klaus C, Hörügel U, Beer M. Tick-borne encephalitis virus (TBEV) infection in horses: Clinical and laboratory findings and epidemiological investigations. Vet Microbiol. 2013;163:368- 72.
- Rushton JO, Lecollinet S, Hubálek Z, et al. Tick-borne encephalitis virus in horses, Austria, 2011. Emerg Infect Dis. 2013;19:635-7.
- Janitza-Futterer D. Serologische Untersuchungen zur endemischen Situation der Infektion mit dem FSME-Virus in einer südbadischen Pferde- und Hundepopulation. Vetmed Diss Munich. 2003
- Sikutova S, Hornok S, Hubalek Z, et al. Serological survey of domestic animals for tick-borne encephalitis and Bhanja viruses in northeastern Hungary. Vet Microbiol. 2009;135:267 -71.
- Sedlák K, Zelená H, Křivda V, Šatrán P. Surveillance of West Nile fever in horses in the Czech Republic from 2011 to 2013. Epidemiol Mikrobiol Immunol. 2014; 63:307-11.
- Cleton NB, van Maanen K, Bergervoet SA, et al. A serological protein microarray for detection of multiple cross-reactive flavivirus infections in horses for veterinary and public health surveillance. Transbound Emerg Dis. 2017; 64:1801–12
- Gresiková M. Excretion of the tick-borne encephalitis virus in the milk of subcutaneously infected cows. Acta Virol. 1958;2:188-92.
- Nosek J, Kozuch O, Ernek E, Lichard M. The importance of goats in the maintenance of tick-borne encephalitis virus in nature. Acta Virol. 1967;11:470-82.
- Böhm B, Schade B, Bauer B, Hoffmann B, Hoffmann D, Ziegler U, Beer M, Klaus C, Weissenböck H, Böttcher J. Tick-borne encephalitis in a naturally infected sheep. BMC Vet Res. 2017;13:267.
- Balogh Z, Egyed I, Ferenczi E, et al. Experimental infection of goats with tick-borne encephalitis virus and possibilities to prevent virus transmission by raw goat milk. Intervirology. 2012;55:194-200.
- Rieille N, Klaus C, Hoffmann D, Péter O, Voordouw M. Goats as sentinel hosts for the detection of tick-borne encephalitis risk areas in the Canton of Valais, Switzerland, BMC Vet Res. 2017;13:217.
- Casati Pagani S, Frigerio Alossa S, Klasu C, Hoffmann D, Beretta O, Bomio-Pacciorini N, Lazzaro M, Merlani G, Ackermann R, Beuret C. First detection of TBE virus in ticks and sero-reactivity in goats in a non-endemic region in the southern part of Switzerland (Canton of Ticino). Ticks Tick Borne Dis. 2019;10:868-874.
- Dorko E, Hockicko J, Rimárová K, Bušová A, Popad’ák P, Popad’áková J, Schréter J. Milk outbreaks of tick-borne encephalitis in Slovakia, 2012-2016. Cent Eur J Public Health 2018;26(Suppl):S47-S50.
- Kerlik J, Avdičová M, Štefkovičová M, Tarkovská V, Pántiková Valachová M, Molčányi T, Mezencev R. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: Analysis of tick-borne encephalitis outbreaks in Slovakia during 2007-2016. Travel Med Infect Dis. 2018;26:37-42.
- Brockmann SO, Oehme R, Buckenmaier T, Beer M, Jeffery-Smith A, Spannenkrebs M, Haag-Milz S, Wagner-Wiening C, Schlegel C, Fritz J, Zange S, Bestehorn M, Lindau A, Hoffmann D, Tiberi S, Mackenstedt U, Dobler G. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Euro Surveill. 2018;23(15):pii=17-00336.
- Cisak E, Wójcik-Fatla A, Zajac V, et al. Prevalence of tick-borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in eastern Poland. Ann Agric Env Med. 2010;17:283-6.
- Paulsen KM, Stuen S, das Neves CG, Suhel F, Gurung D, Soleng A, Stiasny K, Vikse R, Andreassen ÅK, Granquist EG. Tick-borne encephalitis virus in cows and unpasteurized cow milk from Norway. Zoonoses Public Health. 2019;66(2):216-22.
- Klaus C, Ziegler U, Hoffmann D, Press F, Fast C, Beer M. Tick-borne encephalitis virus (TBEV) antibodies in animal sera – occurence in goat flocks in Germany, longevits and ability to recall immunological information after more than six years. BMC Veterinary Res. 2019;15:399.
- Klaus C, Hoffmann B, Moog U, et al. Can goats be used as sentinels for Tick-borne encephalitis (TBE) in non-endemic areas? Experimental studies and epizootiological observations. Berl Münch Tierärztl Wochenschr. 2010;123:441-5.
- Klaus C, Beer M, Saier R, et al. Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus – Epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tick Borne Dis. 2012;3:27-37.
- Klaus C, Hoffmann D, Hoffmann B, Beer M. Frühsommer- Meningoenzephalitis-Virus-Infektionen bei Tieren – Klinik, Diagnostik und epidemiologische Bedeutung. Berl Münch Tierärztl Wochenschr. 2016;130:102-12.
- Vor T, Kiffner C, Hagedorn P, Niedrig M, Ruhe F. Tick burden on European roe deer (Capreolus capreolus). Exp Appl Acarol. 2010;51:405-17.
- Jahfari S, de Vries A, Rijks JM, van Gucht S, Vennema H, Sprong H, Rockx B. Tick-Borne Encephalitis Virus in Ticks and Roe Deer, the Netherlands, Emerg Infect Dis. 2017;23:1028–1030.
- Gerth HJ, Grimshandl D, Stage B, Döller G, Kunz C. Roe deer as sentinels for endemicity of tick-borne encephalitis virus. Epidemiol Infect. 1995;115:355-65.
- Van der Poel WH, Van der Heide R, Bakker D, et al. Attempt to detect evidence for tick-borne encephalitis virus in ticks and mammalian wildlife in the Netherlands. Vector Borne Zoonotic Dis. 2005;5:58-64.
- Skarphédinsson S, Jensen PM, Kristiansen K. Survey of tick-borne infections in Denmark. Emerg Infect Dis. 2005;11:1055-61.
- Kiffner C, Vor T, Hagedorn P, Niedrig M, Rühe F. Determinants of tick-borne encephalitis virus antibody presence in roe deer (Capreolus capreolus) sera. Med Vet Entomol. 2012;26:18-25.
- Ytrehus B, Vainio K, Dudman SG, Gilray J. Tick-borne encephalitis virus and Louping-Ill virus may co-circulate in southern Norway. Vector Borne Zoonotic Dis. 2013;13:762-8.
- Balling A, Plessow U, Beer M, Pfeffer M. Prevalence of antibodies against tick-borne encephalitis virus in wild game from Saxony, Germany. Ticks Tick Borne Dis. 2014;5:805-9.
- Duscher GG, Wetscher M, Baumgartner R. Roe deer sera used for TBE surveillance in Austria. Ticks Tick Borne Dis. 2015;6:489-93.
- Frimmel S, Leister M, Löbermann M, Feldhusen F, Seelmann M, Süss J, Reisinger EC. Seroprevalence of tick-borne-encephalitis virus in wild game in Mecklenburg-Western Pomerania (north-eastern Germany). Ticks Tick Borne Dis. 2016;7:1151–4.
- Roelandt S, Suin V, Van der Stede Y, et al. First TBEV serological screening in Flemish wild boar. Infect Ecol Epidemiol. 2016;6:31099.
- Donoso Mantke O, Escadafal C, Niedrig M, Pfeffer M. Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill. 2011;16:pii=19976.
- Kunze U, the ISW-TBE. Tick-borne encephalitis – still on the map. Report of the 18th annual meeting of the international scientific working group on tick-borne encephalitis (ISW-TBE). Ticks Tick Borne Dis. 2016;7:911-4.
- Bournez L, Umhang G, Faure E, Boucher J-M, Boué F, Jourdain E, Sarasa M, Llorente F, Jiménez-Clavero MA, Moutailler S, Lacour SA, Lecollinet S, Beck C. Exposure of wild ungulates to the Usutu and Tick-borne encephalitis viruses in France in 2009-2014: Evidence of undetected flavivirus circulation a decade ago. viruses 2020;12:10.
- Kríz B, Daniel M, Benes C. The role of game (wild boar and roe deer) in the spread of tick-borne encephalitis in the Czech Republic. Vector Borne Zoonotic Dis. 2014;14:801-7.
- Tonteri E, Jokelainen P, Matala J, Pusenius J, Vapalahti O. Serological evidence of tick-borne encephalitis virus infection in moose and deer in Finland: sentinels for virus circulation. Parasit Vectors. 2016;9:54.
- Paulsen KM, das Neves CG, Granquist EG, Madslien K, Stuen S, Pedersen BN, Viske R, Rocchi M, Laming E, Stiasny K, Andreassen ÅK. Cervids as sentinel-species for tick-borne encephalitis virus in Norway – a serological study. Zoonoses Public Health. 2019 Dec 19;[online ahead of print]
- Bagó Z, Bauder B, Kolodziejek J, Nowotny N, Weissenböck H. Tick-borne encephalitis in a mouflon (Ovis ammon musimon). Vet Rec. 2002;150:218-20.
- Biernat B, Karbowiak G, Stańczak J, Masny A, Werszko J. The first detection of the tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks collected from the lowland European bison (Bison bonasus L.). Acta Parasitologica. 2016;61:130–5.
- Jamsransuren D, Kentaro Y, Hiroaki K, Mitsuhiko A, Kei O, Kei F, et al. Epidemiological survey of tick-borne encephalitis virus infection in wild animals an Hokkaido and Honshu islands, Japan. Japanese J Veterinary Res. 2019;67:163-172.
- Wurm R, Dobler G, Peters M, Kiessig ST. Serological investigations of red foxes (Vulpes vulpes L.) for determination of the spread of tick-borne encephalitis in North Rhine-Westphalia. J Vet Med B Infect Vet Public Health. 2000;47:503-9.
- Rieger MA, Nübling M, Müller W, Hasselhorn HM, Hofmann F. Foxes as indicators for TBE endemicity – a comparative serological and investigation. Zentralbl Bakteriol. 1999;289:610-18.
- Jemeršić L, Dežđek D, Brnić D, Prpić J, Janicki Z, Keros T, Roić B, Slavica A, Terzić S, Konjević D, Beck R. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus elaphus) in Croatia. Ticks Tick Borne Dis. 2014;5:7-13.
- Haemig PD, Lithner S, Sjöstedt de Luna, S, et al. Red fox and tick-borne encephalitis (TBE) in humans: Can predators influence public health. Scand J Infect Dis. 2008;40:527-32.
- Jaenson TGT, Petersson EH, Jaenson DGE, Kindberg J, Pettersson JH, Hjertqvist M, Medlock JM, Bengtsson H. The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: incidence of human TBE correlates with abundance of deer and hares, Parasit Vectors. 2018;11:477.
- Knap N, Avšič-Županc T. Correlation of TBE Incidence with Red Deer and Roe Deer Abundance in Slovenia. PLoS ONE. 2013;8:e66380.
- Rizzoli A, Hauffe HC, Tagliapietra V, Neteler M, Rosà R Forest Structure and Roe Deer Abundance Predict Tick-Borne Encephalitis Risk in Italy. PLoS ONE. 2009;4:e4336.
- Labuda M, Kozuch O, Zuffová E, et al. Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology. 1997; 235:138-43.
- Michelitsch A, Tews BA, Klaus C, Bestehorn-Willmann M, Dobler G, Beers M, Wernike K. In vivo characterization of tick-borne encephalitis virus in bank voles (Myodes glareolus). Viruses. 2019 ;11:1069.
- Imhoff M, Hagedorn P, Schulze Y, et al. Review: Sentinels of tick-borne encephalitis risk. Ticks Tick Borne Dis. 2015;6:592- 600.
- Jääskeläinen A, Tonteri E, Pieninkeroinen I, Sironen T, Voutilainen L, Kuusi M, Vaheri A, Vapalahti O. Siberian subtype tick-borne encephalitis virus in Ixodes ricinus in a newly emerged focus, Finland. Ticks Tick Borne Dis. 2016;7:216-23.
- Smura T, Tonteri E, Jääskeläinen A, vonTroil G, Kuivanen S, Huitu O, Kareinen L, Uusitalo J, Uusitalo R, Hannila-Handelberg T, Voutilainen L, Nikkari S, Sironen T, Sane J, Castén J, Vapalahti O. Recent establishment of tick-borne encephalitisfoci with distinct viral lineages in the Helsinki area, Finland. Emerging Microbes Infect. 2019;8:675-683.
- Knap N, Korva M, Dolinšek V, et al. Patterns of tick-borne encephalitis virus infection in rodents in Slovenia. Zoonotic Dis. 2012;12:236-42.
- Achazi K, Ruzek D, Donoso-Mantke, O, et al. Rodents as Sentinels for the Prevalence of Tick-Borne Encephalitis Virus. Vector Borne Zoonotic Dis. 2011;11: 641-7.
- Pintér R, Madai M, Horváth G, Németh V, Oldal M, Kemenesi G, Dallos B, Bányai K, Jakab F. Molecular detection and phylogenetic analysis of tick-borne encephalitis virus in rodents captured in the transdanubian region of Hungary, Vector Borne Zoonotic Dis. 2014;14:621-4.
- Zöldi V, Papp T, Rigó K, Farkas J, Egyed L. A 4-Year Study of a Natural Tick-Borne Encephalitis Virus Focus in Hungary. 2010–2013, 2015. EcoHealth. 12:174–82.
- Makenov M, Karan L, Shashina N, Akhmentshina M, Zhurenkova O, Kholodilov I, Karganova G, Smirnova N, Grigoreva Y, Yankovskaya Y, Fyodorova M. First detection of tick-borne encephalitis virus in Ixodes ricinus ticks and their rodent hosts in Moscow, Russia. Ticks Tick Borne Dis. 2019;10:101265.
- Tonteri E, Kipar A, Voutilainen L, et al. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus). PLoS ONE. 2013;8:e81214.
- Tonteri E, Jääskeläinen AE, Tikkakoski T, et al. Tick-borne encephalitis virus in wild rodents in winter, Finland (2008- 2009). Emerg Infect Dis. 2011;17:72-5.
- Grzybek M, Tolkacz K, Alsarraf M, Dwużnik D, Szczepaniak K, Tomczuk K, Biernat B, Behnke JM, Bajer A. Seroprevalence of tick-borne encephalitis virus in three species of voles (Microtus spp.) in Poland. J Wildlife Dis. 2020;56;in press.
- Süss J, Gelpi E, Klaus C, et al. Tick-borne encephalitis in naturally exposed monkey (Macaca sylvanus). Emerg Infect Dis. 2007;13:905-7.
- Süss J, Dobler G, Zöller G, et al. Genetic characterization of a tick-borne encephalitis virus isolated from the brain of a naturally exposed monkey (Macaca sylvanus). Int J Med Microbiol. 2008;298(Suppl 44):295-300. doi: 0.1016/j.ijmm.2008.02.001
- Klaus C, Hoffmann B, Beer M, et al. Seroprevalence of tick-borne encephalitis (TBE) in naturally exposed monkeys (Macaca sylvanus) and sheep and prevalence of TBE virus in ticks in a TBE endemic area in Germany. Ticks Tick Borne Dis. 2010;1:141-4.
- Klaus C, Gethmann J, Hoffmann B, et al. Tick infestation in birds and prevalence of pathogens in ticks collected from different places in Germany. Parasitol Res. 2016;115:2729-40.
- Waldenström J, Lundkvist A, Falk KI, et al. Migrating birds and tick-borne encephalitis virus. Emerg Infect Dis. 2007;13:1215- 8.
- Geller J, Nazarova L, Katargina O, et al. Tick-borne pathogens in ticks feeding on migratory passerines in western part of Estonia. Vector Borne Zoonotic Dis. 2013;13:443-8.
- Lommano E, Dvořák C, Vallotton L, Jenni L, Gern L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 2014;5:871-82.
- Kazarina A, Japina K, Keiss O, et al. Detection of tick-borne encephalitis virus in I. ricinus ticks collected from autumn migratory birds in Latvia. Ticks Tick Borne Dis. 2015;6:178-80.
- Csank T, Bhide K, Bencúrová E, Dolinská S, Drzewnioková P, Major P, Korytár Ľ, Bocková E, Bhide M, Pistl J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set, Arch Virol. 2016; 161:1679–1683.
- Hubálek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res. 2012;111:9-36.
- Mikryukova TP, Moskvitina NS, Kononova YV, et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick Borne Dis. 2014;5:145-51.
- ECDC (European Centre for Disease Prevention and Control) Epidemiological situation of tick – borne encephalitis in the European Union and European Free Trade Association countries. Stockholm: ECDC; http://www.ecdc.europa.eu/en/ publications/Publications/TBE-in-EU-EFTA.pdf. 2012.
- Wattle O. Tick-borne encephalitis virusinfektion hos hund. Fördjupninsarbete. Vet Med. Fakulteten, SLU. 1992.
- Müller W. FSME Seroprävalenz beim Hund in Deutschland. Abstract at the 9th InnLab Conference; Munich Germany; 6-8 April 2000; www.alomed.de/DI_innlab.htm.
- Takashima I, Morita K, Chiba M, et al. A case of tick-borne encephalitis in Japan and isolation of the virus. J Clin Microbiol. 1997;35:1943-7.
- Lindblad G. A case of tick-borne encephalitis in a dog. Medlemsblad för Sveriges veterinärforbund. 1960;12:416-17.
- Wandeler A, Steck F, Fankhauser R, et al. Isolierung des Virus der zentraleuropäischen Zeckenenzephalitis in der Schweiz. Path Microbiol. 1972;38: 258-70.
- Reiner B, Fischer A. Frühsommer-Meningoenzephalitis (FSME) beim Hund in Deutschland: Zwei Fallberichte. Kleintierpraxis 1998;43:255-69.
- Ytterberg U, Bjöersdorff A. TBE hos hund – en fallbeskriving. Svensk Vet Tidn. 2002;54:5-7.
- Åblad B. TBE hos en tvåårig hund i Västra Gätaland. Svensk Vet Tidn. 2007;59: 21-3.
- James C. Fästingburen encefalit (TBE) hos hund. Bla Stjärnans Djursjukhus, Göteborg. 2008;1-20.
- Zanoni M, Ortolani DB, Bonilauri P, et al. Encefalite da zecche in un cane. XI Congresso Nazionale S.I.Di.L.V; Parma, Italy; 30 September-2 October 2009;52-53.
- García-Bocanegra I, Jurado-Tarifa E, Cano-Terriza D, Martínez R, Pérez-Marín JE, Lecollinet S. Exposure to West Nile virus and tick-borne encephalitis virus in dogs in Spain. Transbound Emerg Dis. 2018;65:765-772.