E-CDC risk status: endemic
(data as of end 2022)
History and current situation
The first cases of tick-borne encephalitis (TBE) in Estonia were identified in 1949. Today, Estonia is a TBE-endemic country. A TBE-endemic area in Estonia is defined as an area with circulation of the TBEV between ticks and vertebrate hosts as determined by detection of the TBEV or the demonstration of autochthonous infections in humans or animals within the last 20 years.
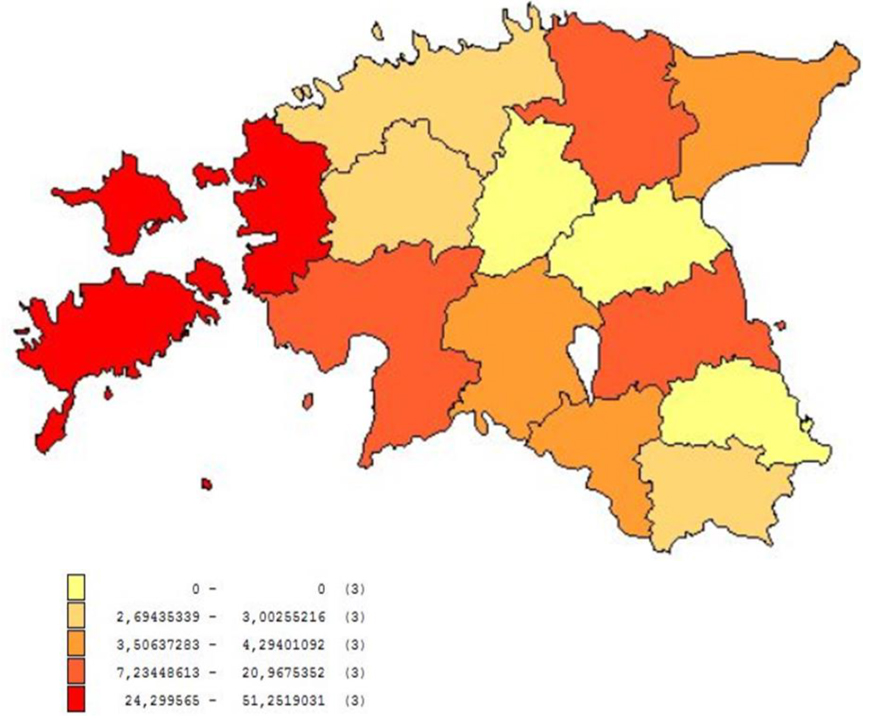
Euro-Asian genotypes of TBEV – the Western or European (TBEV-EU), Siberian (TBEV-Sib), and Far-Eastern (TBEV-FE) subtypes are co-circulating in Estonia. Vectors of TBEV, the tick species Ixodes ricinus and Ixodes persulcatus, are distributed throughout the country. The high-risk season for infection coincides with the period of biological activity of ticks and lasts for 7 months from April to November, peaking in June to August. Most TBE cases are diagnosed in persons ≥60 years of age and the incidence among the rural population is 1.8 times higher than among the urban population.
Overview of TBE in Estonia
| Table 1: Virus, vector, transmission of TBE in Estonia | |
|---|---|
| Viral subtypes, distribution | Co-circulation of European (TBEV-EU), Far-Eastern (TBEV-FE), and Siberian (TBEV-Sib) subtypes |
| Reservoir animals | Rodents, ruminants, game |
| Infected tick species (%) | 2011: I. persulcatus 8%, I. ricinus on mainland 0.6%–0.8% and on Saaremaa island 3.0%. 2013 whole country: I. persulcatus 4.23%, I. ricinus 0.42%. |
| Dairy product transmission | Documented but rare |
| Table 2: TBE reporting and vaccine prevention in Estonia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mandatory TBE reporting | Reporting: neurologists, infectious disease specialist. Case definition
Clinical criteria: a person with symptoms of the central nervous system (meningitis, meningoencephalitis, encephalomyelitis, encephaloradiculitis) Laboratory criteria for case confirmation: At least 1 of the following:
Epidemiological criteria Exposure to a common source (unpasteurized dairy product). Case classification:
Confirmed case: a person meeting the clinical and laboratory criteria for case confirmation. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other TBE surveillance | Laboratory and epidemiological surveillance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special clinical features | Biphasic disease: meningitis, meningoencephalitis, or meningoencephalomyelitis. Risk groups: people who often spend time outdoors (in nature). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Available vaccines | TBE vaccination by age groups in Estonia, 2018-2019
Vaccines available: ENCEPUR CHILDREN and ENCEPUR ADULTS, FSME-IMMUN and FSME-IMMUN junior
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vaccination recommendations and reimbursement | Vaccination recommendations 1998. No reimbursement; self-paid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vaccine uptake by age group/risk group/general population | Vaccine uptake by general population (vaccinated and revaccinated): 2018 – 3.1%; 2019 – 3.7%; 2020 – 3.4%; 2021 – 2.6% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, address/website of TBE National Reference Center | Institute for Health Development, Lab for Virology http://www.tai.ee/en/about-us/national-institute-for-health-development | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
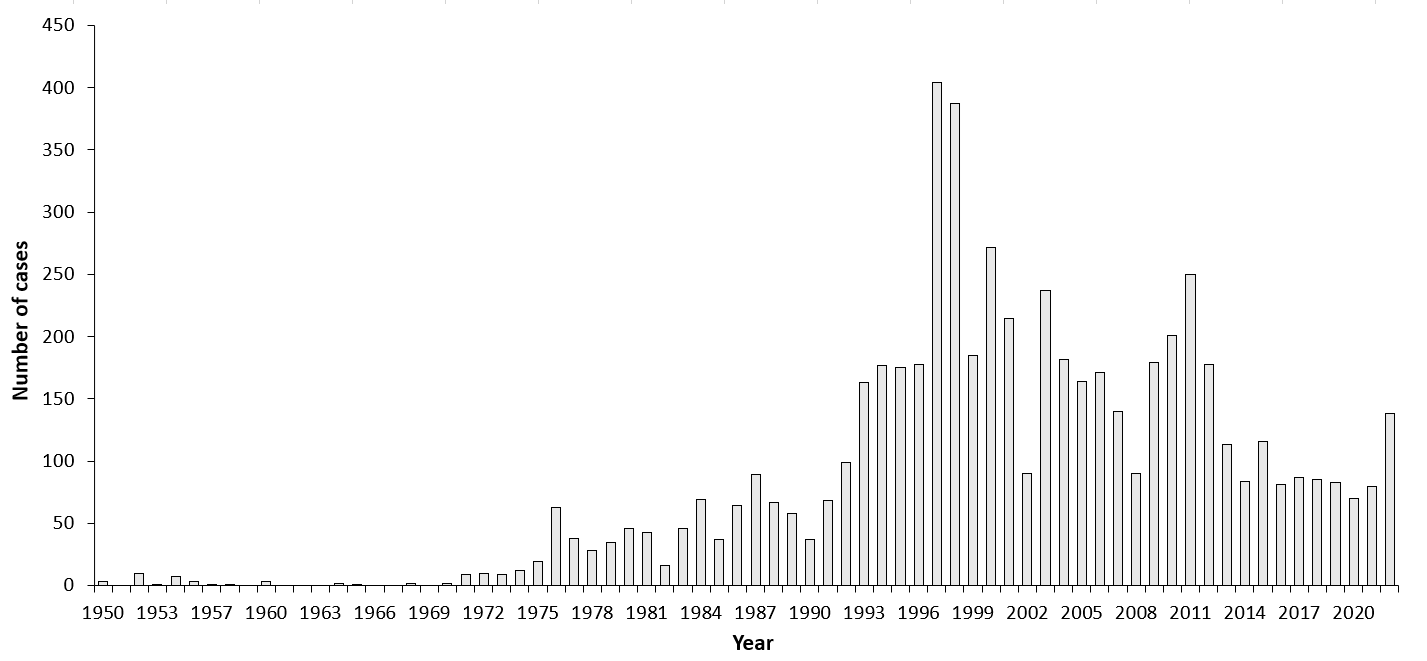
| Years | Number of Cases | Incidence / 105 |
|---|---|---|
| 1950 | 0.3 | 0.3 |
| 1951 | 0 | 0 |
| 1952 | 10 | 0.9 |
| 1953 | 1 | 0.1 |
| 1954 | 7 | 0.6 |
| 1955 | 3 | 0.3 |
| 1957 | 1 | 0.1 |
| 1958 | 1 | 0.1 |
| 1959 | 0 | 0 |
| 1960 | 3 | 0.2 |
| 1961 | 0 | 0 |
| 1962 | 0 | 0 |
| 1963 | 0 | 0 |
| 1964 | 2 | 0.2 |
| 1965 | 1 | 0.1 |
| 1966 | 0 | 0 |
| 1967 | 0 | 0 |
| 1968 | 2 | 0.2 |
| 1969 | 0 | 0 |
| 1970 | 2 | 0.2 |
| 1971 | 9 | 0.7 |
| 1972 | 10 | 0.7 |
| 1973 | 9 | 0.7 |
| 1974 | 12 | 0.8 |
| 1975 | 19 | 1.3 |
| 1976 | 63 | 4.4 |
| 1977 | 38 | 2.6 |
| 1978 | 28 | 1.9 |
| 1979 | 35 | 2.4 |
| 1980 | 46 | 3.1 |
| 1981 | 43 | 2.9 |
| 1982 | 16 | 1.1 |
| 1983 | 46 | 3.0 |
| 1984 | 69 | 4.5 |
| 1985 | 37 | 2.4 |
| 1986 | 64 | 4.1 |
| 1987 | 89 | 5.7 |
| 1988 | 67 | 4.3 |
| 1989 | 58 | 3.7 |
| 1990 | 37 | 2.3 |
| 1991 | 68 | 4.4 |
| 1992 | 99 | 6.5 |
| 1993 | 163 | 10.8 |
| 1994 | 177 | 11.8 |
| 1995 | 175 | 11.8 |
| 1996 | 178 | 12.1 |
| 1997 | 404 | 27.8 |
| 1998 | 387 | 27.0 |
| 1999 | 185 | 12.8 |
| 2000 | 272 | 19.8 |
| 2001 | 215 | 15.8 |
| 2002 | 90 | 6.6 |
| 2003 | 237 | 17.5 |
| 2004 | 182 | 13.5 |
| 2005 | 164 | 12.2 |
| 2006 | 171 | 12.7 |
| 2007 | 140 | 10.4 |
| 2008 | 90 | 6.7 |
| 2009 | 179 | 13.3 |
| 2010 | 201 | 15.0 |
| 2011 | 250 | 18.7 |
| 2012 | 178 | 13.3 |
| 2013 | 113 | 8.5 |
| 2014 | 84 | 6.5 |
| 2015 | 116 | 8.8 |
| 2016 | 81 | 6.2 |
| 2017 | 87 | 6.6 |
| 2018 | 85 | 6.5 |
| 2019 | 83 | 6.3 |
| 2020 | 70 | 5.3 |
| 2021 | 80 | 6.0 |
| 2022 | 138 | 10.4 |
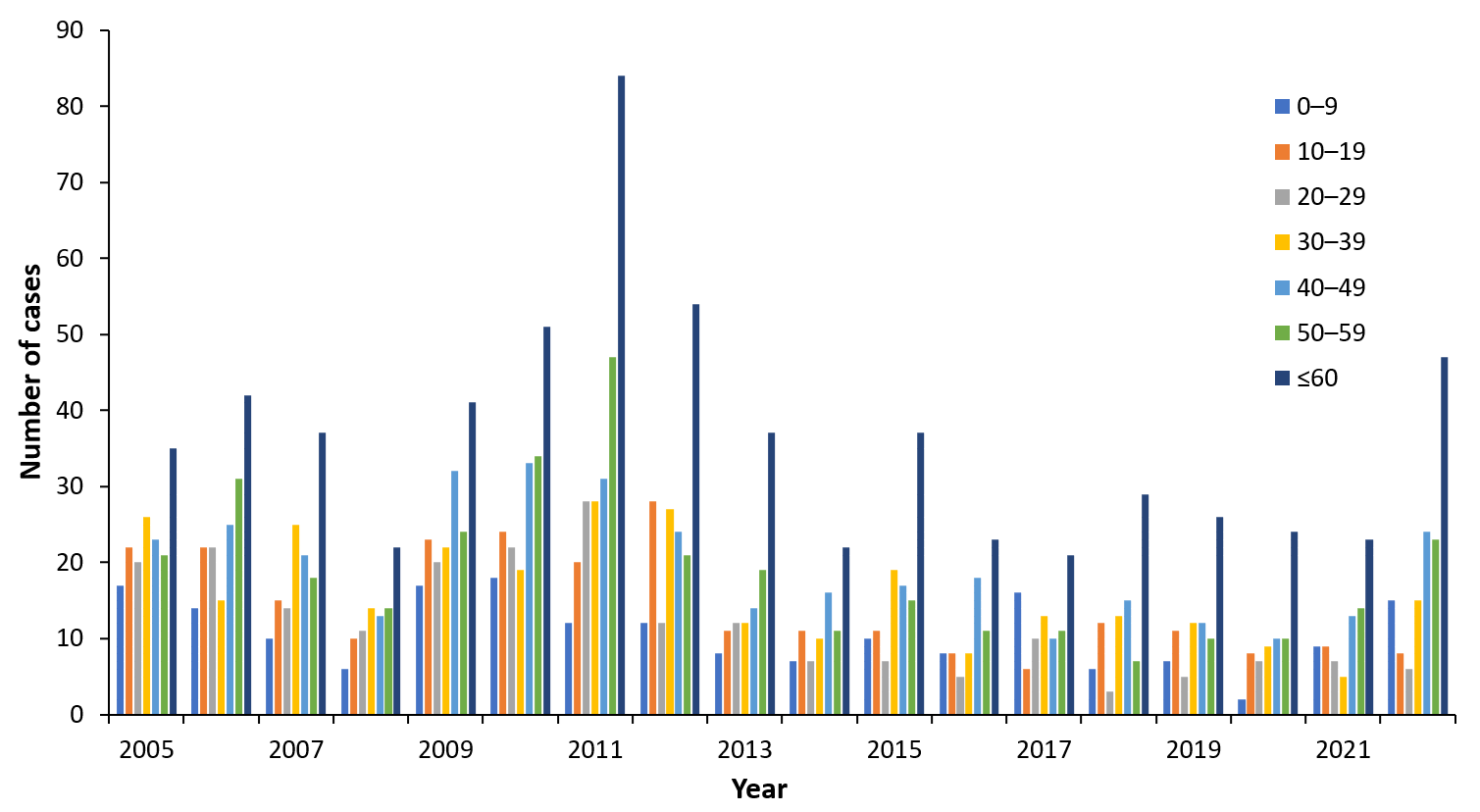
| Year | 0-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60≥ |
|---|---|---|---|---|---|---|---|
| 2005 | 17 | 22 | 20 | 26 | 23 | 21 | 35 |
| 2006 | 14 | 22 | 22 | 15 | 25 | 31 | 42 |
| 2007 | 10 | 15 | 14 | 25 | 21 | 18 | 37 |
| 2008 | 6 | 10 | 11 | 14 | 13 | 14 | 22 |
| 2009 | 17 | 23 | 20 | 22 | 32 | 24 | 41 |
| 2010 | 18 | 24 | 22 | 19 | 33 | 34 | 51 |
| 2011 | 12 | 20 | 28 | 28 | 31 | 47 | 84 |
| 2012 | 12 | 28 | 12 | 27 | 24 | 21 | 54 |
| 2013 | 8 | 11 | 12 | 12 | 14 | 19 | 37 |
| 2014 | 7 | 11 | 7 | 10 | 16 | 11 | 22 |
| 2015 | 10 | 11 | 7 | 19 | 17 | 15 | 37 |
| 2016 | 8 | 8 | 5 | 8 | 18 | 11 | 23 |
| 2017 | 16 | 6 | 10 | 13 | 10 | 11 | 21 |
| 2018 | 6 | 12 | 3 | 13 | 15 | 7 | 29 |
| 2019 | 7 | 11 | 5 | 12 | 12 | 10 | 26 |
| 2020 | 2 | 8 | 7 | 9 | 10 | 10 | 24 |
| 2021 | 9 | 9 | 7 | 5 | 13 | 14 | 23 |
| 2022 | 15 | 8 | 6 | 15 | 24 | 23 | 47 |
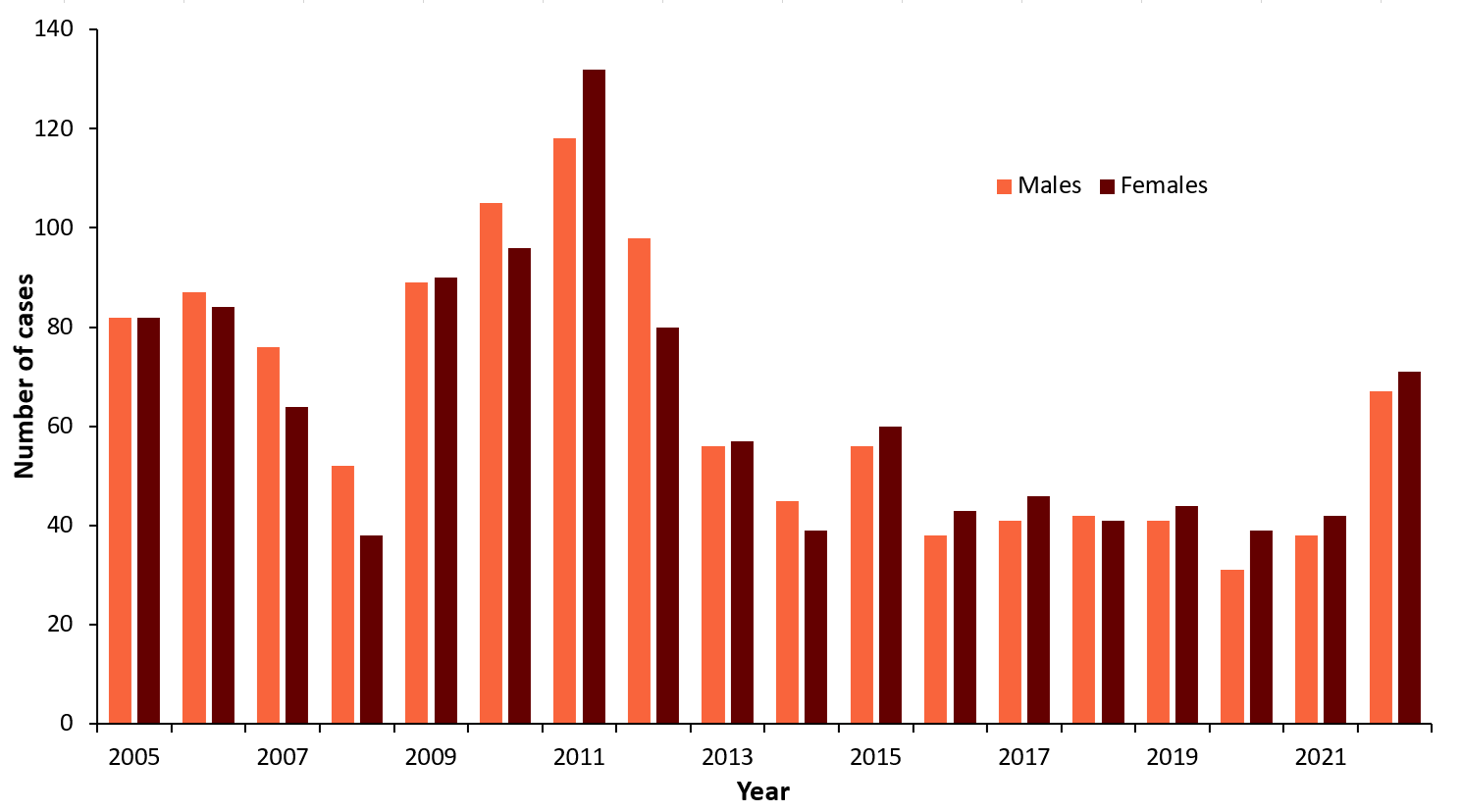
| Men | Women | |
|---|---|---|
| 2005 | 82 | 82 |
| 2006 | 87 | 84 |
| 2007 | 76 | 64 |
| 2008 | 52 | 38 |
| 2009 | 89 | 90 |
| 2010 | 105 | 96 |
| 2011 | 118 | 132 |
| 2012 | 98 | 80 |
| 2013 | 56 | 57 |
| 2014 | 45 | 39 |
| 2015 | 56 | 60 |
| 2016 | 38 | 43 |
| 2017 | 41 | 46 |
| 2018 | 42 | 41 |
| 2019 | 41 | 44 |
| 2020 | 31 | 39 |
| 2021 | 38 | 42 |
| 2022 | 67 | 71 |
Contact:
kkutsar@hotmail.com
Citation:
Kutsar K. TBE in Estonia. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJS, eds. The TBE Book. 6th ed. Singapore: Global Health Press;2023. doi:10.33442/26613980_12b10-6
References
- Health Board. [website] https://www.vaktsineeri.ee/. Immunoprophylaxis of communicable diseases, 2020 report form for vaccinators. Available at https://www.vaktsineeri.ee/et/tervishoiutootajatele-vaktsineerimine. Accessed February 21, 2020.
- Katargina O, Russakova S, Geller J et al. Detection and characterization of tick-borne encephalitis virus in Baltic countries and Eastern Poland. PLoS One. 2013:8(5):e61375