Gerhard Dobler and Ute Mackenstedt
E-CDC risk status: endemic
(data as of end 2022)
History and current situation
The beginning of research on TBE in Germany was influenced and inspired by the results and developments of TBE research in the former Czechoslovakia. There, TBE virus was detected in the Czechoslovak Republic in 1948. In Germany, the first evidence of the presence of TBE virus was found by Sinnecker and his group in the former German Democratic Republic (GDR).1 The first virus strains were isolated also by Sinnecker’s group in the early 1960s.2 In the former Federal Republic of Germany (FRG) TBE research started with research on TBE virus in the region of Franconia by Scheid and Ackermann.3,4 In the region of Lower Franconia a virus was isolated which was called “Zimmern Virus” after the location of the isolation.5 Unfortunately, all these virus strains were lost but it can be assumed that they all belonged to the Western (European) subtype of TBE virus.
In the 1970s, a strong decrease of reported human TBE cases occurred in the formed endemic areas of the German Democratic Republic.6 In Western Germany, only few studies were conducted on the geographic appearance of human TBE cases, mainly led by the company IMMUNO, the first producer of a TBE vaccine in Western Europe. No systematic epidemiological studies are available from this time. TBE was not reportable during this time.
In 2001, TBE became a reportable disease by the new Infection Control Act. From this time on, reliable data on the prevalence of TBE in Germany are available. In the era of molecular detection studies in different areas of Germany on the prevalence of TBE virus in ticks were conducted. In non-engorged ticks the prevalence rates vary depending on the tick stage from 0.1% to 0.5% (nymphs) up to 5% (adult stages).7,8 The molecular characterization of a number of virus strains isolated from ticks in Germany shows that so far all known strains belong to the western (European) subtype of TBE virus.8 Ixodes ricinus, the sheep tick, is the most important vector of TBE virus in Germany. In 2016, TBE virus was detected for the first time in Dermacentor reticulatus in the Federal State of Saxony. In 2016 and 2017 also for the first time in about 50 years two goat milk-borne outbreaks of TBE were registered in Germany (districts of Reutlingen, Tübingen, Baden-Württemberg).
In Germany, TBE is found mainly in the southern part, with the federal states of Bavaria and Baden-Württemberg comprising 80% to 90% of all reported human cases in Germany. There is an increasing number of districts in Saxony, Thuringia and for the first time in 2019 in Lower Saxony which are classified as risk districts by the RKI. The annual reported human cases range from 200 to >550 (RKI, SurvStat). Seroprevalence rates before vaccination programs started in endemic areas in the human population ranged between 3% to 8% with high clustering in some human populations, indicating a highly focal geographic distribution with the endemic areas. Calculating the incidence of the overall German population is generally low (<0.1/100,000), but these figures may give a strongly underestimated risk for some districts in Southern Germany, where the highest incidence rates in Germany can reach incidence rates >10/100,000 in particular districts (e.g., Amberg, Bavaria and Ortenaukreis, Baden-Württemberg).
Overview of TBE in Germany
| Table 1: Virus, vector, transmission of TBE in Germany | |
|---|---|
| Viral subtypes, distribution | European TBEV subtype7,8,13,14 |
| Reservoir animals | Main vertebrate reservoir animals assumed – Myodes glareolus, Apodemus flavicollis, Apodemus agrarius, Apodemus sylvaticus, Microtus agrestis, Microtus arvalis, and Myodes glareolus; detailed information and studies missing.10 |
| Infected tick species (%) | I. ricinus (0.1–5%); D. reticulatus (0.5%). (Chitimia-Dobler et al. submitted; Dobler, personal communication.)16 |
| Dairy product transmission14 | 2016 first outbreak by goat milk and goat cheese for > 50 years in Germany; 2 patients; 2017 outbreak in school with 13 patients (Dobler, personal communication) |
Figure 1: Burden of TBE in Germany over time
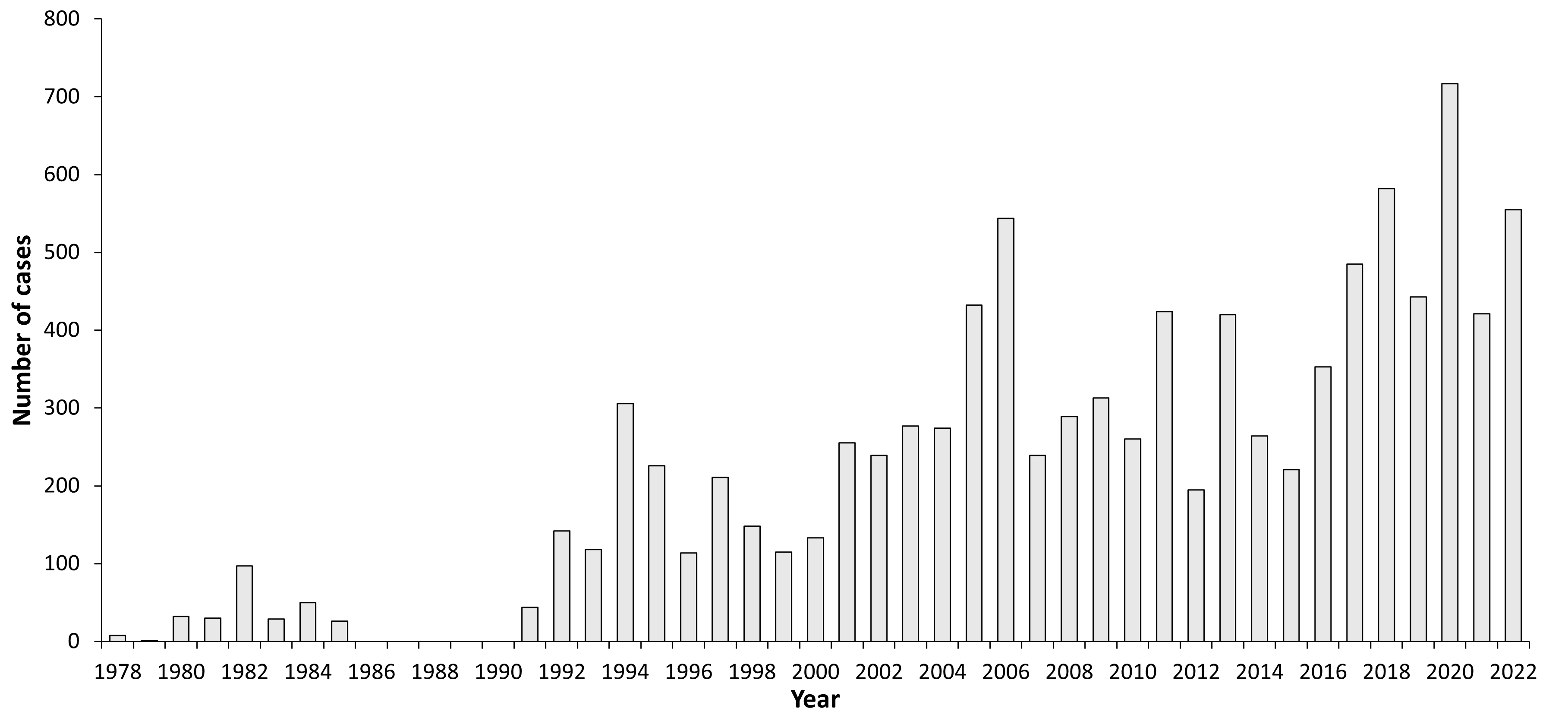
[Robert Koch-Institute, SurvStat. Available at: >http://survstat.rki.de/Content/Query/Create]
| Year | Number of Cases | Incidence / 105 |
|---|---|---|
| 1978 | 8 | |
| 1979 | 1 | <0.1 |
| 1980 | 32 | <0.1 |
| 1981 | 30 | <0.1 |
| 1982 | 97 | 0.17 |
| 1983 | 29 | <0.1 |
| 1984 | 50 | <0.1 |
| 1985 | 26 | <0.1 |
| 1986 | n.a. | |
| 1987 | n.a. | |
| 1988 | n.a. | |
| 1989 | n.a. | |
| 1990 | n.a. | |
| 1991 | 44 | <0.1 |
| 1992 | 142 | 0.18 |
| 1993 | 118 | 0.15 |
| 1994 | 306 | 0.38 |
| 1995 | 226 | 0.28 |
| 1996 | 114 | 0.14 |
| 1997 | 211 | 0.26 |
| 1998 | 148 | 0.18 |
| 1999 | 115 | 0.14 |
| 2000 | 133 | 0.16 |
| 2001 | 255 | 0.31 |
| 2002 | 239 | 0.29 |
| 2003 | 277 | 0.34 |
| 2004 | 274 | 0.33 |
| 2005 | 432 | 0.52 |
| 2006 | 544 | 0.66 |
| 2007 | 239 | 0.29 |
| 2008 | 289 | 0.35 |
| 2009 | 313 | 0.38 |
| 2010 | 260 | 0.32 |
| 2011 | 424 | 0.53 |
| 2012 | 195 | 0.24 |
| 2013 | 420 | 0.53 |
| 2014 | 264 | 0.33 |
| 2015 | 221 | 0.27 |
| 2016 | 353 | 0.43 |
| 2017 | 485 | 0.59 |
| 2018 | 582 | 0.70 |
| 2019 | 443 | 0.53 |
| 2020 | 717 | 0.86 |
| 2021 | 421 | 0.51 |
| 2022 | 555 | 0.66 |
| Table 2: TBE-reporting and vaccine prevention in Germany | |
|---|---|
| Mandatory TBE reporting | All patients with confirmed TBE by serological methods (TBEV IgM ± IgG) or by virus detection are reported to the State Public Health Authorities and to the Federal State Public Health Authority (Robert Koch-Institute: www.rki.de) |
| Other TBE-surveillance | n/a |
| Special clinical features | Biphasic disease in about 50%. Risk groups: permanent inhabitants and visitors of highly endemic areas; mainly acquired during leisure activities |
| 40% of patients meningoencephalitis, 10% meningoencephalomyelitis; No reliable data available on neurological sequelae; in a large study 40%–50% of patients with long-term sequelae; mortality rate 1%–2%9 | |
| Available vaccines | Encepur Erwachsene, Encepur Kinder (Bavarian Nordic), FSME-IMMUN Erwachsene, FSME-IMMUN Kinder (Pfizer) |
| Vaccination recommendations and reimbursement | All inhabitants and visitors of known endemic areas with a risk of tick contact; [STIKO recommendation (www.rki.de)] |
| Vaccine uptake by age group/ risk group/ general population | Vaccination rates in endemic areas 15% to 50%, depending on the district (Survey of the German Society of Consumption Research) |
| Name, address/website of TBE National Reference Center | Robert Koch-Institute (Federal Authority of Public Health), Nordufer 20, 13353 Berlin, Germany (www.rki.de); Bundeswehr Institute of Microbiology, Neuherbergstrasse 11, 80937 München, Germany (gerharddobler@bundeswehr.org). |
Figure 2: Age and gender distribution of TBE in Germany, 2022
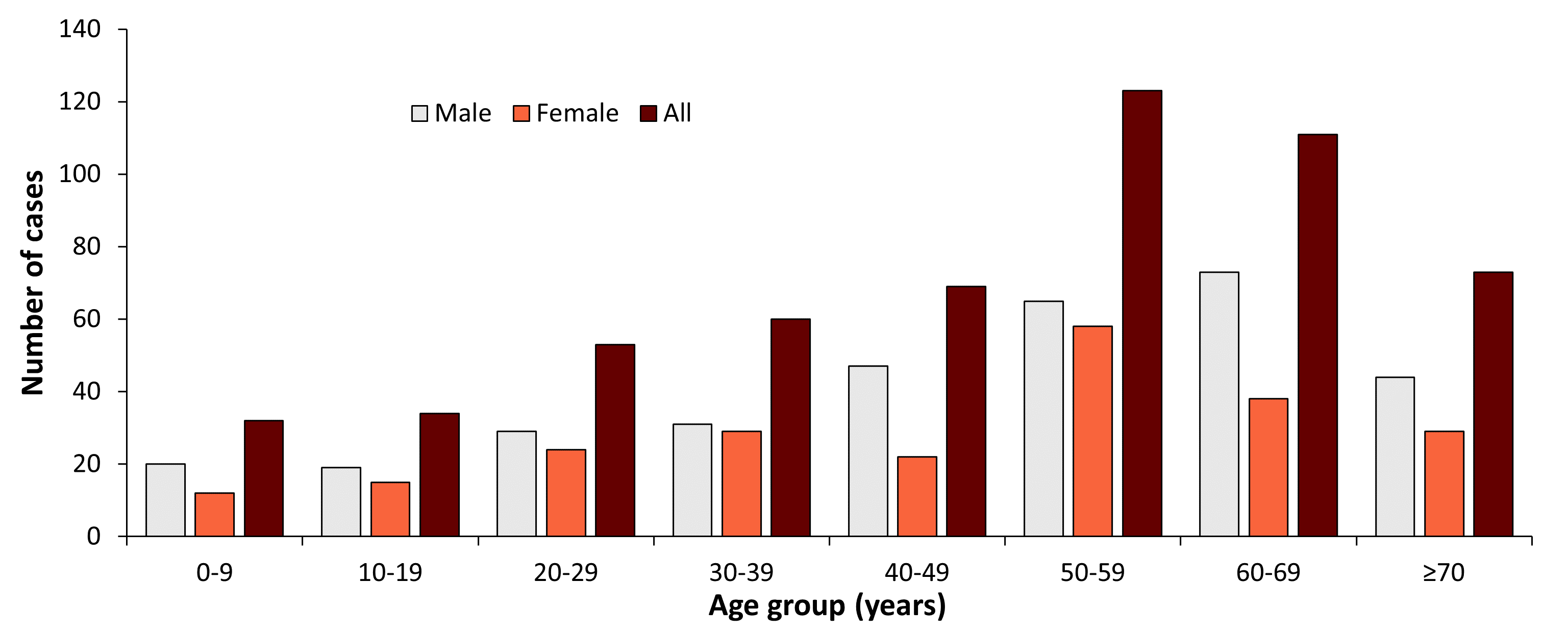
| 2010 | ||||
|---|---|---|---|---|
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 3 | 6 | 9 | |
| 10-19 | 12 | 4 | 16 | |
| 20-29 | 13 | 7 | 20 | |
| 30-39 | 18 | 16 | 34 | |
| 40-49 | 39 | 28 | 67 | |
| 50-59 | 26 | 24 | 50 | |
| 60-69 | 26 | 8 | 34 | |
| >70 | 23 | 7 | 30 | |
| 2011 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 19 | 7 | 25 | |
| 10-19 | 19 | 13 | 1 | 33 |
| 20-29 | 18 | 8 | 26 | |
| 30-39 | 15 | 23 | 38 | |
| 40-49 | 76 | 42 | 118 | |
| 50-59 | 62 | 25 | 87 | |
| 60-69 | 34 | 18 | 52 | |
| >70 | 27 | 18 | 45 | |
| 2012 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 3 | 3 | 6 | |
| 10-19 | 5 | 3 | 8 | |
| 20-29 | 10 | 9 | 19 | |
| 30-39 | 14 | 7 | 21 | |
| 40-49 | 34 | 15 | 49 | |
| 50-59 | 27 | 19 | 46 | |
| 60-69 | 13 | 7 | 20 | |
| >70 | 17 | 9 | 26 | |
| 2013 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 17 | 5 | 22 | |
| 10-19 | 22 | 5 | 27 | |
| 20-29 | 25 | 15 | 40 | |
| 30-39 | 26 | 24 | 1 | 51 |
| 40-49 | 47 | 36 | 83 | |
| 50-59 | 53 | 35 | 88 | |
| 60-69 | 33 | 17 | 50 | |
| >70 | 38 | 21 | 59 | |
| 2014 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 5 | 4 | 9 | |
| 10-19 | 5 | 3 | 8 | |
| 20-29 | 11 | 8 | 19 | |
| 30-39 | 17 | 14 | 31 | |
| 40-49 | 39 | 24 | 63 | |
| 50-59 | 39 | 20 | 59 | |
| 60-69 | 25 | 10 | 35 | |
| >70 | 27 | 13 | 40 | |
| 2015 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 5 | 4 | 9 | |
| 10-19 | 11 | 5 | 16 | |
| 20-29 | 11 | 6 | 17 | |
| 30-39 | 11 | 6 | 17 | |
| 40-49 | 17 | 23 | 40 | |
| 50-59 | 30 | 21 | 51 | |
| 60-69 | 27 | 12 | 39 | |
| >70 | 18 | 14 | 32 | |
| 2016 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 14 | 6 | 20 | |
| 10-19 | 16 | 8 | 24 | |
| 20-29 | 18 | 11 | 29 | |
| 30-39 | 18 | 14 | 32 | |
| 40-49 | 25 | 32 | 57 | |
| 50-59 | 35 | 50 | 85 | |
| 60-69 | 48 | 19 | 67 | |
| >70 | 28 | 11 | 39 | |
| 2017 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 13 | 7 | 20 | |
| 10-19 | 14 | 14 | 28 | |
| 20-29 | 22 | 13 | 33 | |
| 30-39 | 36 | 16 | 52 | |
| 40-49 | 43 | 27 | 70 | |
| 50-59 | 81 | 52 | 1 | 134 |
| 60-69 | 52 | 25 | 77 | |
| >70 | 50 | 19 | 69 | |
| 2018 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 25 | 15 | 40 | |
| 10-19 | 16 | 11 | 27 | |
| 20-29 | 34 | 15 | 49 | |
| 30-39 | 30 | 27 | 57 | |
| 40-49 | 57 | 42 | 99 | |
| 50-59 | 73 | 48 | 1 | 123 |
| 60-69 | 68 | 28 | 96 | |
| >70 | 66 | 25 | 91 | |
| 2019 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 16 | 4 | 20 | |
| 10-19 | 19 | 6 | 25 | |
| 20-29 | 23 | 14 | 37 | |
| 30-39 | 26 | 15 | 41 | |
| 40-49 | 39 | 29 | 68 | |
| 50-59 | 58 | 48 | 106 | |
| 60-69 | 47 | 37 | 84 | |
| >70 | 43 | 20 | 63 | |
| 2020 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 28 | 13 | 41 | |
| 10-19 | 31 | 20 | 51 | |
| 20-29 | 38 | 18 | 56 | |
| 30-39 | 41 | 28 | 69 | |
| 40-49 | 50 | 33 | 83 | |
| 50-59 | 102 | 80 | 182 | |
| 60-69 | 76 | 51 | 1 | 128 |
| >70 | 75 | 28 | 103 | |
| 2021 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 16 | 6 | 22 | |
| 10-19 | 21 | 3 | 24 | |
| 20-29 | 19 | 10 | 1 | 30 |
| 30-39 | 30 | 19 | 49 | |
| 40-49 | 31 | 17 | 48 | |
| 50-59 | 59 | 49 | 108 | |
| 60-69 | 48 | 24 | 72 | |
| >70 | 38 | 27 | 63 | |
| 2022 | ||||
| Age Group (years) | Males | Females | Unknown | All |
| 0-9 | 20 | 12 | 32 | |
| 10-19 | 19 | 15 | 34 | |
| 20-29 | 29 | 24 | 53 | |
| 30-39 | 31 | 29 | 60 | |
| 40-49 | 47 | 22 | 69 | |
| 50-59 | 65 | 58 | 123 | |
| 60-69 | 73 | 38 | 111 | |
| >70 | 44 | 29 | 73 |
TBEV-isolation and TBE cases in Germany
| Year of isolation | Strain name | Source of isolation | Location of isolation |
|---|---|---|---|
| 197511 | K23 | Tick | Karlsruhe, Baden-Württemberg |
| 20068 | AS33 | Tick | Amberg, Bavaria |
| 200712 | Salem | Monkey brain | Salem, Baden-Württemberg |
| 2009* | HM strains | Tick | Amberg, Bavaria |
| 201113 | HB171/11 | Tick | Heselbach, Bavaria |
| 2014** | Bottnang | Tick | Stuttgart, Baden-Württemberg |
| 2016* | HM-M1 | Bank vole brain | Amberg, Bavaria |
| 2016*,** | tbd | Tick | Aubachstrasse, Baden-Württemberg |
| 201615 | tbd | Tick | Aubachstrasse, Baden-Württemberg |
| 201715 | tbd | Tick | Schiltach, Baden-Württemberg |
| 201716 | Tick (D. reticulatus) | Battaune, Saxony |
Contact:
gerharddobler@bundeswehr.org
Citation:
Dobler G, Mackenstedt U. TBE in Germany. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press;2023. doi:10.33442/26613980_12b13-6
References
- Sinnecker H. Zeckenenzephalitis in Deutschland. Zentralbl Bakteriol Orig. 1960;180:12-18.
- Apitzsch L, Sinnecker H, Wigand R, Berndt D. ZE-Virusisolierungen in der DDR 1965/66 und einige Stammdifferenzen. Zentralbl Bakteriol Orig. 1968;207:429-34.
- Queisser H. Beobachtungen über verschiedene Fälle von Zeckenenzephalitis in Deutschland. Münch Med Wochenschr. 1962;47:2288.
- Scheid W, Ackermann R, Bloedhorn H, Löser R, Liedtke G, Skrtic N. Untersuchungen über das Vorkommen der Zentraleuropäischen Enzephalitis in Süddeutschland. Dtsch Med Wochenschr. 1964;89:2313-7.
- Ackermann R, Scheid W, Küpper B. Infektionen mit dem Virus der Zentraleuropäischen Enzephalitis in Südwest-Deutschland. Dtsch Med Wochenschr. 1966;91(25):1141-3.
- Süss J, Sinnecker H, Sinnecker R, Berndt D, Zilske E, Dedek G, Apitzsch L: Epidemiology and ecology of tick-borne encephalitis in the eastern part of Germany between 1960 and 1990 and studies on the dynamics of a natural focus of tick-borne encephalitis. Zentralbl Bakteriol. 1992;277(2):224-35.
- Süss J, Beziat P, Rohr HP, Treib J, Haass A. Detection of the tick-borne encephalitis virus (TBEV) in ticks in several federal “Länder” of Germany by means of the polymerase chain reaction (PCR) – characterization of the virus. Infection. 1996;24:403-4.
- Kupča AM, Essbauer S, Zoeller G, de Mendonça PG, Brey R, Rinder M, Pfister K, Spiegel M, Doerrbecker B, Pfeffer M, Dobler G. Isolation and molecular characterization of a tick-borne encephalitis virus strain from a new tick-borne encephalitis focus with severe cases in bavaria, Germany. Ticks Tick Borne Dis. 2010;1(1):44-51.
- Kaiser R. Rick-borne encephalitis: clinical findings and prognosis in adults. Wien Med Wochenschr. 2012;162(11-12):239-43.
- Achazi K, Růžek D, Donoso-Mantke O, Schlegel M, Ali HS, Wenk M, Schmidt-Chanasit J, Ohlmeyer L, Rühe F, Vor T, Kiffner C, Kallies R, Ulrich RG, Niedrig M. Rodents as sentinels for the prevalence of tick-borne encephlitis virus. Vector Borne Zoonotic Dis. 2011;11(6):641-7.
- Heinz FX, Tuma W, Kunz C. Antigenic and immunogenic properties of defined physical forms of tick-borne encephalitis virus structural proteins. Infect Immun. 1981;33(1):250-7.
- Süss J, Dobler G, Zöller G, Essbauer S, Pfeffer M, Klaus C, Liebler-Tenorio EM, Gelpi E, Stark B, Hotzel H. Genetic characterization of a tick-borne encephalitis virus isolated from the brain of a naturally exposed monkey (Macaca sylvanus). Int J Med Microbiol. 2008;298(S1):295-300.
- Dobler G, Bestehorn M, Antwerpen M, Överby-Wernstedt A. Complete genome sequence of a low-virulence tick-borne encephalitis virus strain. Genome Announc. 2016;4(5):e01145-16.
- Brockmann SO, Oehme R, Buckenmaier T, Beer M, Jeffery-Smith A, Spannenkrebs M, Haag-Milz S, Wagner-Wiening C, Schlegel C, Fritz J, Zange S, Bestehorn M, Lindau A, Hoffmann D, Tiberi S, Mackenstedt U, Dobler G. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurized goat milk and cheese in Germany, May 2016. Euro Surveill. 2018;23(15).
- Bestehorn M, Weigold S, Kern WV, Chitimia-Dobler L, Mackenstedt U, Dobler G, Borde JP. Phylogenetics of tick-borne encephalitis virus in endemic foci in the upper Rhine region in France and Germany. PLoS One. 2018;13(10):e0204790.
- Chitimia-Dobler L, Lemhöfer G, Krol N, Bestehorn M, Dobler G, Pfeffer M. Repeated isolation of tick-borne encephalitis virus from Dermacentor reticulatus ticks in an endemic area in Germany. Parasit Vectors. 2019;12(1):90.