Dace Zavadska and Zane Freimane
E-CDC risk status: endemic
(data as of end 2022)
History and current situation
Aggregated data on TBE cases in Latvia are available from 1955,1 but serological testing for TBE began in the 1970s.2 Since TBE became notifiable in Latvia, epidemiological changes of disease incidence have been dramatic. Between 1990–2000 Latvia had the highest rates of TBE incidence in the world, ranging from 8 to 53 cases per 100,000 population.2 Although the incidence decreased significantly in the past 10 years to about half – from 14.58/100,000 in 2010 to 7.86/100,000 in 2018 – Latvia still ranks very high among all countries in Europe with an annual incidence of 12.67/100,000 in 2022. The distribution of TBE cases in Latvia varies between different regions with the highest incidence usually registered near the northwestern coast.
The Centre for Disease Prevention and Control (CDPC) of Latvia is the governmental institution that provides TBE surveillance in Latvia. Based on national legislation, there is countrywide mandatory but passive case-based reporting, guided by case definition of the European Centre for Disease Prevention and Control (ECDC) since 2012. Adoption of the standardized European case definition for TBE ensures a more specific capture of TBE cases as well as the impact by vaccination.
The main vectors of the TBE virus in Latvia are ticks of the family Ixodidae, mainly Ixodes ricinus and Ixodes persulcatus in the eastern part of the country.3 All three main TBEV subtypes are carried by ticks in Latvia – the European, Siberian and Far-Eastern subtype.4,5,6 Epidemiological investigations suggests that in Latvia, ticks carry a higher TBEV load than in other at-risk countries, and moreover, up to 20%–40% of ticks are infected in highly endemic areas.7 Latvia also has one the highest reported rates of TBEV transmission via unpasteurized dairy products, mainly goat milk,2 which accounts for 0.5%–3.5% of all cases (2011–2019).
The largest recent study of the epidemiology of TBE in Latvia documents on a population basis with active case search in hospitals that mostly persons in the age group 18–59 years are affected, mostly males. This is in line with the general risk factors for TBE, i.e. active lifestyle with increased outdoor activities, travelling, and other factors that increase the risk of tick-human contact.8 Children (0–17 years) in Latvia make up 5.6% of all TBE cases.
The most common clinical manifestation of TBE was meningitis, with the highest number of cases in the age group 18–59 years. For children, meningitis was also the most frequent cause of hospitalization.9 Compared to other age groups, more severe TBE clinical forms (meningoencephalitis, etc.) were mainly reported among the age group >60 years.
Vaccination remains the most effective protective measure against TBE.10,11,12 In Latvia, there is only a partial National Immunization Program, which has provided vaccine free of charge for children living in highly endemic areas since 2006 and orphans/children without parental care in the whole country since 2010. Vaccination is mandatory for employees with a high risk of occupational exposure such as forest workers, military personnel, and lab workers and it is paid by the employer. For other residents of Latvia and travelers vaccination is strongly recommended but not reimbursed, however most private insurance companies cover TBE vaccine expenses.13,14 Because of the National Immunization Program for children, TBE vaccine uptake in children reached up to 77% in highly endemic areas and 22% nationwide, reducing the proportion of TBE cases among children from 12.5% in 2001 to 3.6% in 201015 and 2016. Vaccine uptake in the whole population vaccination was 39% in 200915 and it increased to 52.5% in 2015.16
Currently used vaccines in Latvia are FSME-Immun® (TicoVac, used since 1995) and Encepur® (since 2001 for adults and 2002 for children). FSME-Immun® is the most commonly used TBE vaccine in Latvia, with an up to 86% market share in those who had received at least one dose where the brand administered was captured.17 In the future, uptake data need to be carefully monitored in order to explain epidemiological findings.
Overview of TBE in Latvia
| Table 1: Virus, vector, transmission of TBE in Latvia | |
|---|---|
| Viral subtypes, distribution | In Latvia, all 3 main TBEV sub-types circulate: European, Siberian, and Far Eastern In Latvia 1-96 is a close relative to the Vasilchenko strain (Siberian sub-type), and RK1424 is related to the Sofjin strain (Far Eastern sub-type).4,5,6 |
| Reservoir animals | Among the small rodents identified in the most long-term I.ricinus monitoring site (Riga region) in 1997-2001 were Clethrionomys glareolus (85%), followed by Sorex araneus, Apodemus flavicollis and Apodemus agrarius.19 |
| Infected tick species (%) | Ixodes ricinus ticks are spread in the western and central part of Latvia, and in small numbers also in the eastern part of the country. Ixodes persulcatus dominates only in the eastern part of the country, comprising 58%–99% of all collected ticks. Earlier data reveals that TBEV annual prevalence from 1993 to 2002 in the field-collected adults for I. ricinus adults varied between 1.7% and 26.6% and for I. persulcatus – between 0% and 37.3%. The infection level in ticks removed from humans was much higher and from 1998 to 2002 reached about 30%.3,6,7 |
| Dairy product transmission | Rare |
| Table 2: TBE reporting and vaccine prevention in Latvia | |
|---|---|
| Mandatory TBE reporting | Mandatory notification since 1955. Based on national legislation, there is countrywide mandatory case-based passive reporting and the European Centre for Disease Prevention and Control (ECDC) case definition for TBE was adopted in Cabinet Regulations in 2012. Aggregated data on TBE cases are available from 1955 and case-based data in electronic format are available from 2007. Prior to 2012, the case definition of TBE in Latvia included (1) hospitalization because of central nervous system disease and (2) confirmation of infection with TBE virus by laboratory diagnosis, usually by the demonstration of specific IgM antibodies by ELISA.3,20 |
| Other TBE-surveillance | None |
| Special clinical features | Study done in Children’s Clinical university hospital reveals that Biphasic fever course was presented in 50% (n=41) of children treated in the hospital between 2000–2015.9 |
| Annual mortality varies from 0% to 1.3% (1973–2009) and is not related to the overall incidence of TBE. Follow-up for 1–13 years of a cohort of 100 patients revealed long-term sequelae in over 50%, more commonly in those suffering focal forms of acute TBE.3 | |
| Available vaccines21,22 | TicoVac (0.25 and 0.5 ml) since 1995 (FSME-Immun)
|
| Vaccination recommendations and reimbursement | There is only a partial National Immunization Program in place which has recommended vaccination for children and adolescents living in endemic areas since 2007 and has provided vaccine free of charge for children living in highly endemic areas since 2006 and orphans/children without parental care in the whole country since 2010. Vaccination is mandatory for high-risk groups and/or those with high occupational exposure such as forest workers, military personnel, and lab workers and is paid by the employer. Vaccination is also recommended, but not reimbursed for adults. Also most insurance companies cover TBE vaccination costs.16,23(https://likumi.lv/doc.php?id=11215 Cabinet Regulations Nr.330. Vaccination regulations) |
| Vaccine uptake by age group/ risk group/ general population | The vaccination uptake overall was 53% in 2015.* In Latvia, approximately 22% of children had been vaccinated by the end of 2010, most (77%) of whom were living in highly endemic areas, the cost of which was reimbursed by the state. The vaccination rate for the national population was 39% in 2009 and 41% in 2010.17,23 |
| Name, address/website of TBE National Reference Center | Center of Disease Prevention and Control of Latvia – www.spkc.gov.lv; Address: Duntes iela 22, k-5, Rīga, Latvija, LV 1005) Diagnostics: Latvian Centre of Infectious Diseases (Riga East University Hospital) – https://www.aslimnica.lv/en/saturs/latvian-centre-infectious-diseases Address: 3 Linezera Street, Riga, LV-1006 |
Figure 1: Burden of TBE in Latvia over time8
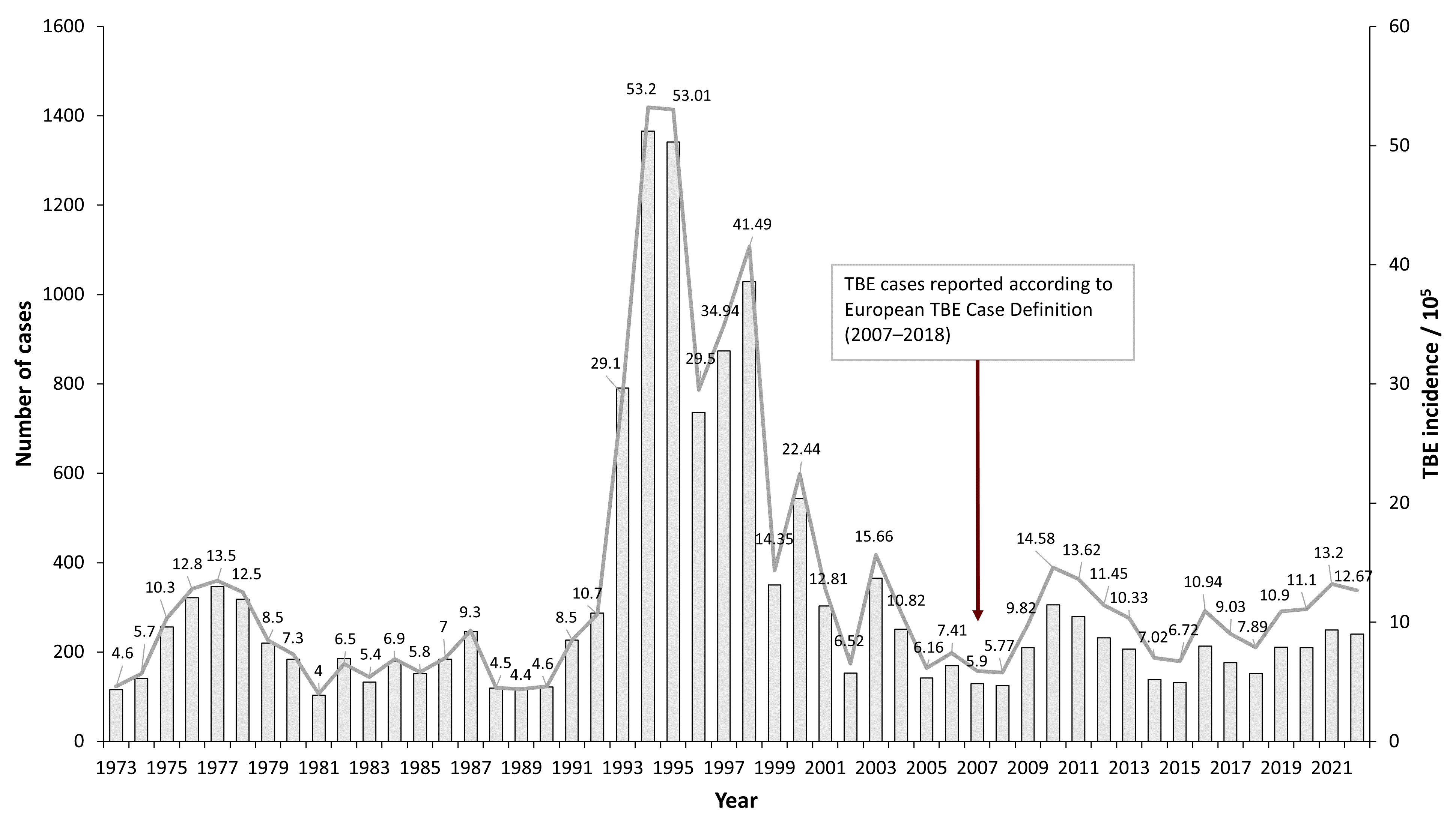
| Year | Number of Cases (according to European Case Definition)* | Incidence / 105 |
|---|---|---|
| 1973 | 116 | 4.6 |
| 1974 | 141 | 5.7 |
| 1975 | 256 | 10.3 |
| 1976 | 322 | 12.8 |
| 1977 | 347 | 13.5 |
| 1978 | 318 | 12.5 |
| 1979 | 220 | 8.5 |
| 1980 | 184 | 7.3 |
| 1981 | 103 | 4 |
| 1982 | 186 | 6.5 |
| 1983 | 133 | 5.4 |
| 1984 | 179 | 6.9 |
| 1985 | 152 | 5.8 |
| 1986 | 184 | 7 |
| 1987 | 246 | 9.3 |
| 1988 | 119 | 4.5 |
| 1989 | 117 | 4.4 |
| 1990 | 122 | 4.6 |
| 1991 | 227 | 8.5 |
| 1992 | 287 | 10.7 |
| 1993 | 791 | 29.1 |
| 1994 | 1366 | 53.2 |
| 1995 | 1341 | 53.01 |
| 1996 | 736 | 29.5 |
| 1997 | 874 | 34.94 |
| 1998 | 1029 | 41.49 |
| 1999 | 350 | 14.35 |
| 2000 | 544 | 22.44 |
| 2001 | 303 | 12.81 |
| 2002 | 153 | 6.52 |
| 2003 | 365 | 15.66 |
| 2004 | 251 | 10.82 |
| 2005 | 142 | 6.16 |
| 2006 | 170 | 7.41 |
| 2007 | 129 | 5.90 |
| 2008 | 125 | 5.77 |
| 2009 | 210 | 9.82 |
| 2010 | 306 | 14.58 |
| 2011 | 280 | 13.62 |
| 2012 | 232 | 11.45 |
| 2013 | 207 | 10.33 |
| 2014 | 139 | 7.02 |
| 2015 | 132 | 6.72 |
| 2016 | 213 | 10.94 |
| 2017 | 176 | 9.03 |
| 2018 | 152 | 7.86 |
| 2019 | 211 | 10.9 |
| 2020 | 210 | 11.1 |
| 2021 | 249 | 13.2 |
| 2022 | 240 | 12.67 |
Figure 2: Age and gender distribution of TBE in Latvia (2007–2016, n=1973)8
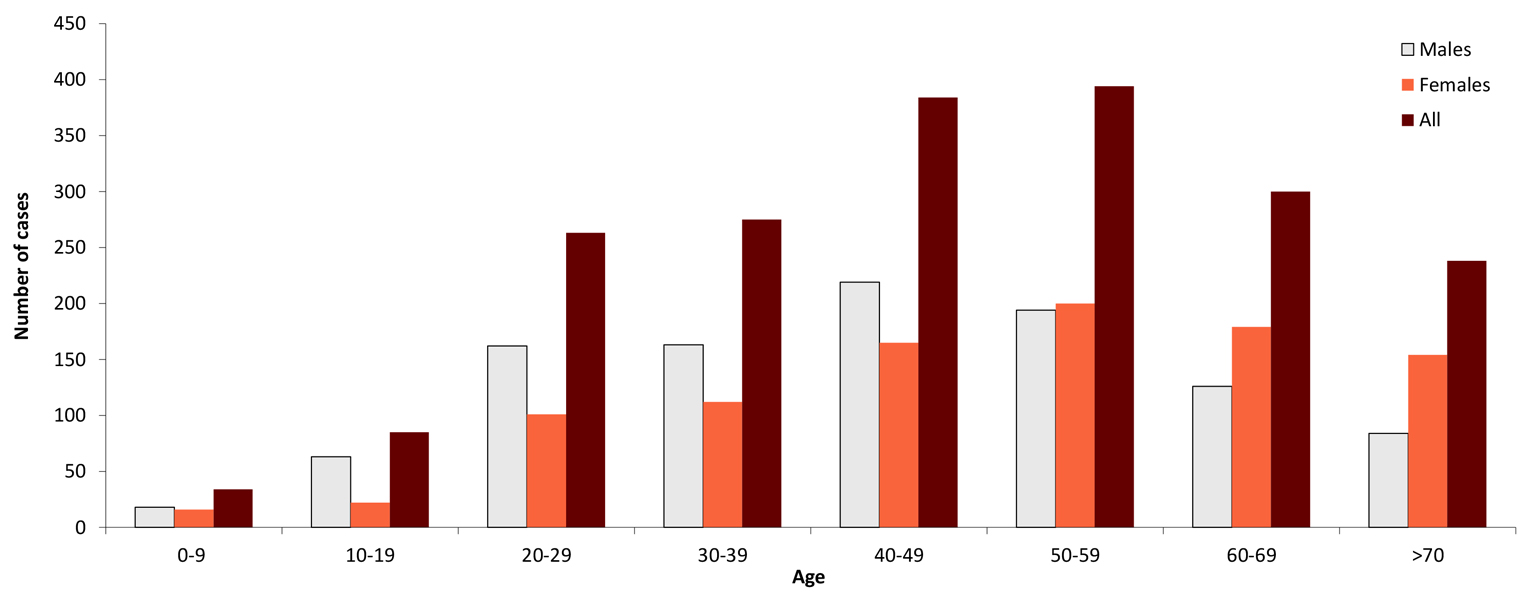
| Age group (years) | Males | Females | All |
|---|---|---|---|
| 0-9 | 18 | 16 | 34 |
| 10-19 | 63 | 22 | 85 |
| 20-29 | 162 | 101 | 263 |
| 30-39 | 163 | 112 | 275 |
| 40-49 | 219 | 165 | 384 |
| 50-59 | 194 | 200 | 394 |
| 60-69 | 126 | 179 | 300 |
| >70 | 84 | 154 | 238 |
Figure 3: TBEV-isolation and TBE cases in Latvia (2007–2016, n=1973)8
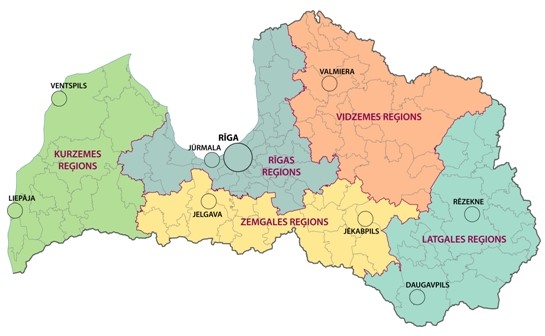
Figure 4: Burden of TBE (“CNS disease”) by 5 regions of Latvia (2007–2016, n=1973)8
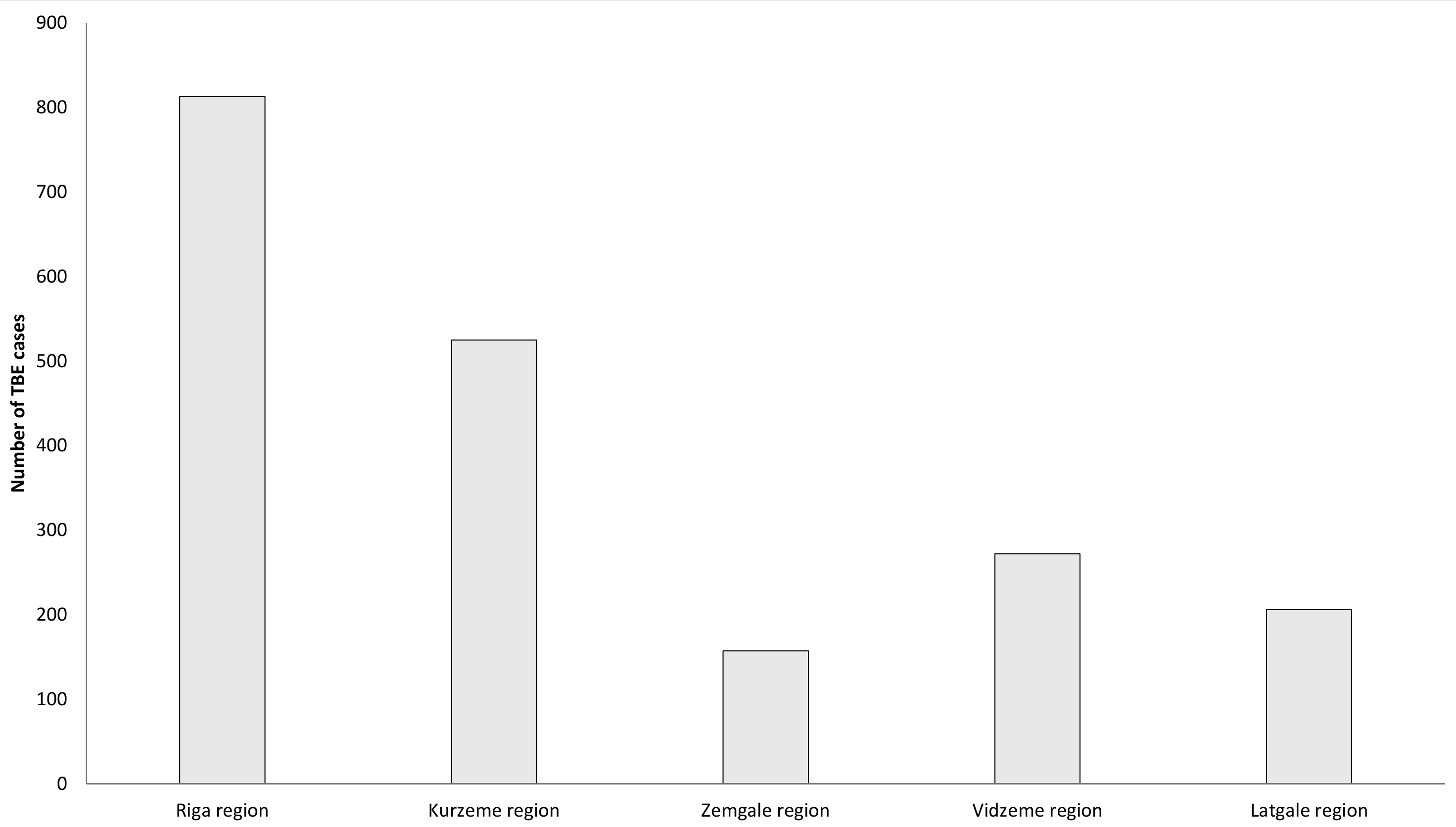
| Region of Latvia | Number of Cases | Incidence / 105 |
|---|---|---|
| Riga region | 813 | 12.25 |
| Kurzeme region | 525 | 19.34 |
| Zemgale region | 157 | 6.14 |
| Vidzeme region | 272 | 12.79 |
| Latgale region | 206 | 6.76 |
Figure 5: Regions of Latvia18
Contact:
dzavadska@apollo.lv
Citation:
Zavadska D, Freimane Z. TBE in Latvia. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi:10.33442/26613980_12b19-6
References
- Lucenko I, Jansone I, Velicko I, Pujate E. Tick-borne encephalitis in Latvia. Euro Surveill. 2004;(26):pii=2495.
- Sumilo D, Bormane A, Vasilenko V, Golovljova I, Asokliene L, Zygutiene M, Randolph S. Upsurge of tick-borne encephalitis in the Baltic States at the time of political transition, independent of changes in public health practices. Clin Microbiol Infect. 2009;15(1):75-80.
- Karelis G, Bormane A, Logina I, Lucenko I, Suna N, Krumina A, Donaghy M. Tick-borne encephalitis in Latvia 1973-2009: epidemiology, clinical features and sequelae. Eur J Neurol. 2012;19(1):62-8.
- Süss J, Schrader C, Abel U, Bormane A, Duks A, Kalnina V. Characterization of tick-borne encephalitis (TBE) foci in Germany and Latvia (1997-2000). Int J Med Microbiol. 2002;291 Suppl 33:34-42.
- Mavtchoutko, V., Vene, S., Haglund, M., Forsgren, M., Duks, A., Kalnina, V., Hörling, J., Lundkvist, A. Characterization of tick-borne encephalitis virus from Latvia. J Med Virol. 2000;60:216-22.
- Bormane A, Zeltiņa A, Lucenko I, Mavčutko V, Duks A, Pujate E, Ranka R, Baumanis V. Tick-borne encephalitis – pathogen, vectors and epidemiological situation in Latvia 2002-2003. Acta Universitatis Latviensis. 2004;670:27-37.
- Bormane A, Lucenko I, Duks A, Mavtchoutko V, Ranka R, Salmina K, Baumanis V. Vectors of tick-borne diseases and epidemiological situation in Latvia in 1993-2002. Int J Med Microbiol. 2004;293 Suppl 37:36-47.
- Zavadska D, Odzelevica Z, Karelis G, Liepina L, Litauniece ZA, Bormane A, et al. (2018) Tick-borne encephalitis: A 43-year summary of epidemiological and clinical data from Latvia (1973 to 2016). PLoS One. 2018;13(11):e0204844. doi:10.1371/journal.pone.0204844
- Zavadska D, Odzelevica Z. “Tick-borne encephalitis in Children’s Clinical University Hospital – clinical course and neurological outcome” 2015, presented at 18th meeting ISW-TBE 2016.
- World Health Organization. Tick-borne encephalitis. 2015. [online] – [Reference 20.01.17.] Available: http://www.who.int/biologicals/vaccines/tick_borne_encephalitis/en/
- World Health Organization Position Paper. Vaccines against tick-borne encephalitis. 2011. [online] – [Reference 01.03.17.] Available: http://www.who.int/immunization/sage/1_TBE_PP_Draft_13_Mar_2011_SAGE_apr_2011.pdf
- Heinz F, Stiasny K, Holzmann H, Vitek M, Kriz B, Essl A, Kundi M. Vaccination and Tick-borne Encephalitis, Central Europe. Emerg Infect Dis. 2013;19(1):69-76.
- Republic of Latvia. Cabinet Regulations No. 330. Vaccination regulations. 2000.g. [online] – [reference 15 February 2017] Available: http://likumi.lv/doc.php?id=11215
- The Centre for Disease Prevention and Control of Latvia.[online] – [reference 15.02.17.] Available: https://spkc.gov.lv/lv/tavai-veselibai/infekcijas-slimibas/vakcinacija/pret-ercu-encefalitu-berniem
- Zavadska D, Anca I, André F, Bakir M, Chlibek R, Čižman M, Ivaskeviciene I, Mangarov A, Mészner Z, Pokorn M, Prymula R, Richter D, Salman N, Šimurka P, Tamm E, Tešović G, Urbancikova I, Usonis V. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother. 2013;9(2):362-74.
- Zavadska, D. Latvia: Success Story of TBE Vaccination. Presentation for 15th ISW-TBE. 2013.
- TBE Compliance & Coverage Country Report – Latvia; Report prepared for Pfizer by GfK SE. GfK SE. October 2015
- Ministry of Environmental Protection and Regional Development of the Republic of Latvia. [online]. Available: http://www.varam.gov.lv/lat/darbibas_veidi/reg_att/pl_reg/?doc=13637
- Bormane A (2007) Ixodes ricinus L. un Ixodes persulcatus P. Sch. (Acari: Ixodidae) izplatība, to pārnēsāto infekcijas slimību nozīme un molekulārā epidemioloǵija Latvijā. Dissertation, University of Latvia
- Dumpis U, Crook D, Oksi J. Tick-borne encephalitis. Clin Infect Dis. 1999;28(4):882-90.
- State Agency of Medicines of Latvia, 2017
- Sumilo D, Asokliene L, Avsic-Zupanc T, Bormane A, Vasilenko V, Lucenko I, Golovljova I, Randolph SE. Behavioural responses to perceived risk of tick-borne encephalitis: vaccination and avoidance in the Baltics and Slovenia. Vaccine. 2008;19;26(21):2580-8.
- Zavadska et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother. 2013